Is Periodontitis and Rheumatoid Arthritis Inter-Related?
Received: 04-Mar-2018 / Accepted Date: 09-Apr-2018 / Published Date: 17-Apr-2018 DOI: 10.4172/2376-032X.1000231
Abstract
There is a growing awareness of the link between periodontal and systemic inflammatory conditions, such as RA. Inflammation plays a key role in the origin of RA, its chronification, and in a progression of the disease with soft and hard tissue destruction, in a way similar to the situation seen in patients with chronic periodontitis.
While the existing level of evidence is low, the association may be reflective of common underlying dysregulation of an inflammatory response in these individuals, and it seems that a decrease in periodontal inflammation in some way influences the level of systemic inflammation, and appears to contribute to the clinical improvement of the disease.
Keywords: Rheumatoid arthritis; Periodontitis; Citrullinated protein; Inflammation
Introduction
Periodontal disease is a multifactorial, complex disease where there is an interplay of host- tissue response and bacterial infection. Any shift of the equilibrium towards the latter, causes periodontal tissue destruction and is characterized by loss of connective tissue attachment. Periodontal disease are not only a threat to dentition, but may also be a threat to general heath. There are reports suggesting increased prevalence of diabetes, atherosclerosis, myocardial infarction, stroke and rheumatic arthritis (RA) in patients with periodontal disease [1,2] There is a growing awareness of the link between periodontal and systemic inflammatory conditions, such as RA and coronary artery disease based on common etiopathogenic mechanisms [2-5] All forms of inflammatory periodontal diseases are associated with chronic inflammation , resulting in the destruction of periodontal ligament and bone [5]. In addition to other chronic condition of altered connective tissue metabolism ,hormone imbalance and altered immune function have likewise been associated with increased risk of periodontal disease [6].
Rheumatoid arthritis is an autoimmune disease characterized by chronic inflammation of the joints, particularly the small joints of the hands and feet, with progressive destruction resulting in variable degrees of deformity and functional disability. Inflammation plays a key role in the origin of RA, its chronification, and in progression of the disease with soft and hard tissue destruction, in a way similar to the situation seen in patients with chronic periodontitis [7].
A number of hypotheses have tried to explain the relationship between PD and RA. The currently most widely accepted hypothesis is the “double hit model”, which postulates that a first “hit” in the form of inflammation due to periodontitis is followed by a second “hit” at joint level, with an exacerbation of the inflammatory response in these locations. Another hypothesis points to the development of an autoimmune response generated by proteins partially altered by enzymes of bacterial origin, such as anti-citrullinated protein antibodies in RA [8-10].
Common Risk Factor Between Periodontitis and Rheumatoid Arthritis
Periodontitis and rheumatoid arthritis (RA) are both chronic inflammatory diseases & share numerous features including tissue and bone destruction; production of inflammatory mediators including cytokines, prostaglandins and degradation enzymes (i.e. MMPs) and common risk factors such as smoking [11,12]. The destruction of soft and hard tissue is a result of a large number of signal molecules released by inflammatory cells in the synovia/gingiva which contribute, directly or indirectly, to the degradation of tissue and bone [13-15]. Both diseases have similar cytokine profiles characterized by high levels of inflammatory mediators (such as PGE2, TNF-, IL-6 and IL-1β) and degradation enzymes (e.g. MMP1, MMP9 and MMP13), and decreased levels of anti-inflammatory mediators (such as IL-10 and TGF-β) [15-20] In addition, a well-established major risk factor for both periodontitis and RA is cigarette smoking. Higher frequency of bone loss, attachment loss and edentulism is exhibited in smokers, compared with non-smokers. Moreover, a well-established genetic risk factor for RA, HLA-DRB1 SE alleles, has also been implicated as a risk factor for periodontitis [21-23]. Additionally, antibodies associated with RA (both ACPA and RF) have been detected in non-RA patients with periodontitis [12]. For example, individuals with periodontitis have higher serum ACPA-levels compared to healthy controls. Furthermore, increased levels of citrullinated proteins have been reported in inflamed periodontal tissue, and expression of ACPAs has been demonstrated in gingival crevicular fluid (GCF) and saliva of patients with RA [24,25]. Similarly, RF has been reported in gingiva, subgingival plaques and in serum of patients with periodontitis [12].
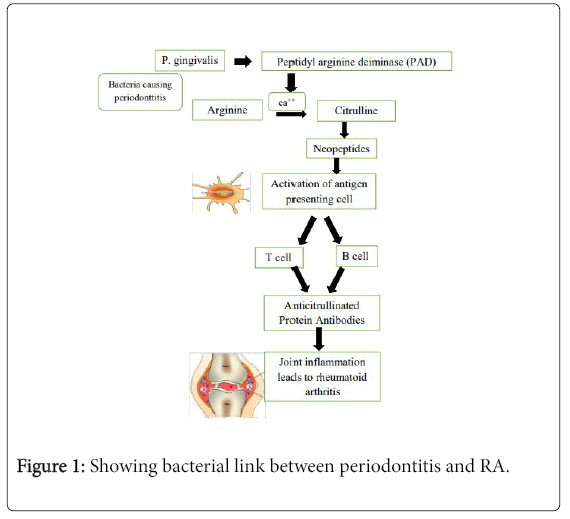
Bacterial Link Between Periodontitis And Rheumatoid Arthritis [12,26]
In 2004, a hypothesis of a possible pathogenic connection between periodontitis and RA was proposed, implicating the involvement of the periodontal pathogen P. gingivalis in the pathogenesis of RA, through the process of citrullination. Citrullination is an enzyme-mediated post-translational modification of the amino acid arginine in a protein into the non-standard amino acid citrulline (Figure 1).
Laboratory Tests In Common Between Rheumatoid Arthritis And Periodontitis [27]
There is no single test that can assess and predict the status of RA and periodontal disease. However, the combination of clinical and laboratory markers give more meaningful measures of disease activity and severity than a single test. Laboratory markers such as levels of rheumatoid factors, prostaglandins, collagen degradation products and C- reactive protein are altered in inflammatory conditions such as periodontitis and RA. Accordingly, a multitude of factors including clinical parameters, immunopathology and microbiology should be considered in order to reach an acceptable diagnosis and predictive ability for both RA and periodontal disease.
Periodontal Treatment and Disease Activity
Regarding the possible relationship between periodontal treatment and disease activity, it has been suggested that the control of periodontal disease could contribute to lessen infection and periodontal inflammation by adopting preventive measures with good oral hygiene, supragingival and subgingival scaling and root planing. These measures could reduce the clinical activity of RA, with a decrease in the serum levels of certain products derived from the inflammatory process [28] Systematic review indicates that the application of conservative treatments clearly improves the periodontal parameters (bleeding upon probing, pocket depth and attachment loss) in patients with RA and chronic periodontal disease [29] Furthermore, this periodontal improvement was seen to be associated to beneficial effects in relation to other disease assessment parameters such as the DAS28 score and ESR [28-30]. In this regard, ESR decreased significantly – suggesting a reduction in systemic inflammation following nonsurgical periodontal treatment. The DAS 28 score showed a tendency towards lessened signs and symptoms of disease, with a decrease in the number of painful joints, and lesser morning stiffness and joint effusion, independently of the drugs used to treat RA.
Although Pinho et al. [31] found the difference between the studied groups to be significant at three months of follow up, significance was lost after 6 months. The follow up period in the different studies ranged from 6 weeks to 6 months, and might be too short to detect clinical changes referred to periodontal infection and inflammation.
Conclusion
While the existing level of evidence is low, the association may be reflective of common underlying disregulation of inflammatory response in these individuals, and it seems that a decrease in periodontal inflammation in some way influences the level of systemic inflammation, and appears to contribute to clinical improvement of the disease.
However, further randomized controlled clinical trials including a larger number of patients, with appropriate blinding and longer periods of follow-up are necessary to confirm this finding.
References
- Scannapieco FA (1998) Position paper of The American Academy of Periodontology: Periodontal disease as a potential risk factor for systemic diseases. J Periodontol 69: 841-850.
- Beck JD, Garcia RG, Heiss G, Vokonas PS, Offenbacher S (1996) Periodontal disease and cardiovascular disease. J Periodontol 67: 1123-1137.
- Offenbacher S, Katz VL, Fertik GS (1996) Periodontal infection as a risk factor for pre-term low birth weight. J Periodontol 67: 1103-1113.
- Saini R (2009) Periodontitis is true infection. J Glob Infect Dis 1: 149-151.
- Ebsersole JL (1990) Systemic humoral immune response in periodontal disease. Crit Rev Oral Biol Med 1: 283-331.
- Bartold PM (1991) Connective tissues of the periodontium. Research and clinical implications. Aus ral dent J 36: 522-261.
- Weyand CM (2000) New insights into the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 39: 3-8.
- Sakkas LI, Bogdanos DP, Katsiari C, Platsoucas CD (2014) Anti-citrullined peptides as autoantigens in rheumatoid arthritis- relevance to treatment. Autoimmun Rev 13: 114-20.
- Konig MF, Paracha AS, Moni M, Bingham CO, Andrade F (2015) Defining the role of Porphyromonas gingivalis peptidylarginine deiminase (PPAD) in rheumatoid arthritis through the study of PPAD biology. Ann Rheum Dis 74: 2054-2061.
- Mikuls TR, Paune JB, Yu F, Thiele GM, Reynolds RJ, et al. (2014) Periodontitis and porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Reumatol 66: 1090-100.
- de Pablo P, Chapple IL, Buckley CD, Dietrich T (2009) Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol 5: 218-224.
- Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G (2004) Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation 28: 311-318.
- Burrage PS, Mix KS, Brinckerhoff CE (2006) Matrix metalloproteinases: Role in arthritis. Front Biosci 11: 529-543.
- Bartold PM, Marino V, Cantley M, Haynes DR (2010) Effect of Porphyromonas gingivalis-induced inflammation on the development of rheumatoid arthritis. J Clin Periodontol 37: 405-411.
- Bascones A, Noronha S, Gomez M, Mota P, Gonzalez Moles MA, et al. (2005) Tissue destruction in periodontitis: Bacteria or cytokines fault? Quintessence Int 36: 299-306.
- Kobayashi T, Yoshie H (2015) Host responses in the link between periodontitis and rheumatoid arthritis. Curr Oral Health Rep 2: 1-8.
- Keyszer G, Lambiri I, Nagel R, Keysser C, Keysser M, et al. (1999) Circulating levels of matrix metalloproteinases MMP-3 and MMP-1, tissue inhibitor of metalloproteinases 1 (TIMP-1), and MMP-1/TIMP-1 complex in rheumatic disease. Correlation with clinical activity of rheumatoid arthritis versus other surrogate markers. J Rheumatol 26: 251-258.
- Seguier S, Gogly B, Bodineau A, Godeau G, Brousse N (2001) Is collagen breakdown during periodontitis linked to inflammatory cells and expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival tissue? J Periodontol 72: 1398-406.
- Araki Y, Mimura T (2017) Matrix metalloproteinase gene activation resulting from disordred epigenetic mechanisms in rheumatoid arthritis. Int J Mol Sci 18: 905.
- Franco C, Patricia HR, Timo S, Claudia B, Marcela H (2017) Matrix metalloproteinases as regulators of periodontal inflammation. Int J Mol Sci 18: E440.
- Bonfil JJ, Dillier FL, Mercier P, Reviron D, Foti B, et al. (1999) A "case control" study on the role of HLA DR4 in severe periodontitis and rapidly progressive periodontitis. Identification of types and subtypes using molecular biology (PCR.SSO). J Clin Periodontol 26: 77-84.
- Katz J, Goultschin J, Benoliel R, Brautbar C (1987) Human leukocyte antigen (HLA) DR4. Positive association with rapidly progressing periodontitis. J Periodontol 58: 607-610.
- Marotte H, Farge P, Gaudin P, Alexandre C, Mougin B, et al. (2006) The association between periodontal disease and joint destruction in rheumatoid arthritis extends the link between the HLA-DR shared epitope and severity of bone destruction. Ann Rheum Dis 65: 905-909.
- Harvey GP, Fitzsimmons TR, Dhamarpatni AA, Marchant C, Haynes DR, et al. (2013) Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and 64 anti-citrullinated protein antibodies in human gingiva. J Periodontal Res 48: 252-261.
- Nesse W, Westra J, van der Wal JE, Abbas F, Nicholas AP, et al. (2012) The periodontium of periodontitis patients contains citrullinated proteins which may play a role in ACPA anti-citrullinated protein antibody formation. J Clin Periodontol 39: 599-607.
- Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, et al. (2010) Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum 62: 2662-2672.
- Kaur S, White S, Bartold PM (2013) Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res 92: 399-408.
- Ortiz P, Bissada NF, Palomo L, Han YW, Al-Zahrani MS, et al. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol 80: 535-540.
- Ranade SB, Doiphode S (2012) Is there a relationship between periodontitis and rheumatoid arthritis? J Indian Soc Periodontol 16: 22-27.
- Okada M, Kobayashi T, Ito S, Yokoyama T, Abe A, et al. (2013) Periodontal treatment decreases levels of antibodies to Prophyromonas gingivalis and citrulline in patients with reumatoid arthritis and periodontitis. J Periodontol 84: e74-84.
- Pinho MN, Oliveira RD, Novaes AB Jr, Voltarelli JC (2009) Relationship between periodontitis and rheumatoid arthritis and the effect of non-surgical periodontal treatment. Braz Dent J 20: 355-364.
Citation: Indurkar MS, Bhailume PS, Raut AS (2018) Is Periodontitis and Rheumatoid Arthritis Inter-Related?. J Interdiscipl Med Dent Sci 6: 231. DOI: 10.4172/2376-032X.1000231
Copyright: © 2018 Indurkar MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 8076
- [From(publication date): 0-2018 - Dec 06, 2023]
- Breakdown by view type
- HTML page views: 7254
- PDF downloads: 822