PSMC2 Knockdown Inhibits Multiple Myeloma Cell Proliferation and Enhances Apoptosis
Manuscript No. CMB-22-51956 / Editor assigned: 22-Jan-2022 / PreQC No. CMB-22-51956 (PQ) / Reviewed: 23-Feb-2022 / QC No. CMB-22-51956 / Revised: 28-Feb-2022 / Manuscript No. CMB-22-51956 (R) / Accepted Date: 28-Feb-2022 / Published Date: 07-Mar-2022 DOI: 10.4172/1165-158X.1000226
Abstract
Proteasome 26S subunit ATPase 2 (PSMC2) has been identified as being potentially related to certain human cancers. However, the expression levels and functional importance of PSMC2 in multiple myeloma are still uncertain. PSMC2 expression in the levels of mRNA and protein was detected by qRT-PCR and western blot assay. The present study concentrated on clarifying the significance of PSMC2 on multiple myeloma cell behaviors including proliferation, migration and apoptosis by the CCK8 assay, the transwell assay and the flow cytometry. PSMC2 knockdown caused by RNA interference in multiple myeloma cell lines would significantly suppress cell proliferation, migration, enhance apoptosis and arrest cell cycle. Our results reflected that PSMC2 knockdown could inhibit multiple myeloma cell proliferation and enhance apoptosis and that the inhibition of PSMC2 might be a considerable therapeutic strategy for the treatment of multiple myeloma.
Keywords
Multiple myeloma; PSMC2; Cell proliferation; Cell apoptosis; Cell migration
Introduction
Multiple myeloma (MM) is an incurable type of hematological malignancy that originates in bone marrow and is characterized by clonal proliferation of plasma cells [1,2]. To our best knowledge, the current therapies of MM include initial therapy [3], autologous stem cell transplantation [4], consolidation therapy [5], maintenance therapy [6] as well as treatment of relapse [7]. Moreover, these treatments have markedly improved the median overall survival of patients, but patients still suffer from poor prognosis and most patients will inevitably relapse [8]. In the past few years, the treatment of relapsed patients with MM improved due to the introduction of pomalidomide [9], immune-modifying drugs [10], monoclonal antibodies [11], the histone deacetylase inhibitor Panobinostat [12] and new-generation proteasome inhibitors carfilzomib and ixazomib [13]. To be exactly, the proteasome inhibitors bortezomib, carfilzomib, and ixazomib were approved by the FDA for the treatment of MM, revealing the feasibility of proteasome as an anti-tumor target [14]. However, the three inhibitors are all covalent inhibitors, this combination mode may cause poor specificity and severe side effects, eventually drug resistance evolving limits their therapeutic success [15]. Therefore, new proteasome inhibitors were urgently needed to overcome the above problems.
The ubiquitin-proteasome pathway is mainly characterized by participating in the selective degradation of proteins in eukaryotic cells [16]. The 26S proteasome is one of the well-known multimeric proteases, which is absolutely essential for the regulation of cell quality and the destruction of intracellular regulatory proteins such as transcription factors and cell cycle regulators [17]. Beyond that, 26S proteasome is consisted of the 19S regulatory particles and the 20S proteasome catalytic particle [18]. There are three different catalytic subunits in the 20S proteasome, namely β5, β2 and β1, which were reported to contribute to the degradation of tumor suppressor proteins, especially β5 subunit [19]. Therefore, considerable research has been devoted to the development of proteasome inhibitors for cancer therapy. For example, several potential metal complexes based on copper, manganese and cadmium have been discovered as proteasome inhibitors and are widely explored as one of the important cancer treatment strategies [20]. Proteasome 26S subunit, ATPase 2 (PSMC2), located in 7q22.1-q22.3 in the genome, is a pivotal member of the 19S regulatory subunit of the 26S proteasome [21]. Additionally, as free 20S particle, but not 19S particle, is available in cells, it indicates that 26S proteasome assembly is limited by the level of 19S regulatory subunit [22]. As such, PSMC2 is an indispensable member in the assembly process of 19S and 26S proteasome [23]. Recently, there are many excellent reviews in the literature dealing with the relationship between PSMC2 with human cancers. In detail, the levels of PSMC2 in osteosarcoma and pancreatic cancer were highly expressed, which involved in the initiation and progression of cancer by promoting cancer cell proliferation and inhibiting apoptosis [24,25]. Although PSMC2 was regarded as a novel therapeutic target relevant to human cancers, the functional validation for PSMC2 in multiple myeloma is completely unclear. Here, we found that PSMC2 was highly expressed in multiple myeloma cells. At the same time, we demonstrated the effects of PSMC2 on multiple myeloma cell proliferation, migration and apoptosis, which may be a novel therapeutic target for multiple myeloma treatment in the future.
Materials and Methods
Cell lines and cell culture
The human multiple myeloma cell lines (MM.1, MM.1S and RPMI 8226) used in this study were provided by Cell Resource Center, Shanghai Academy of Life Sciences, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in 1640 medium with 10% FBS and maintained at 37℃ in 5% CO2. It should be emphasized to change the medium every 72 h.
Lentivirus RNAi construction and transfection
Using PSMC2 as a template, three RNAi target sequences were designed. The sequence with the highest knockdown efficiency was selected and ligated to the linearized vector BR-V-108 through the restriction sites at both ends, and the product was transferred to the prepared DH5α E. coli competent cells. Positive clones were identified by PCR and the plasmids were extracted by the Endofree Maxi plasmid kit. The three plasmids BR-V108, BR-V307 and BR-V112 were used to co-transfect 293T cells, the cells were harvested after transfection for 48-72 h. Finally, the cells were cultured for 72 h at 37℃ and the transfection efficiency was evaluated according to the expression of green fluorescent protein (GFP).
RNA extraction and qRT-PCR
The cells were collected and the total RNA was extracted with TRIzol reagent (Sigma, St. Louis, MO, USA) according to the manufacturer’s instruction and cDNA was obtained by using the Promega M-MLV Kit (Promega Corporation, Madison, Wisconsin, USA). Real-time quantitative PCR system was performed with SYBR Green Mastermixs Kit (Vazyme, Nanjing, Jiangsu, China). GAPDH was chosen as an internal control and the relative expression of RNA was calculated according to the 2-△△Ct method. The sequences of primers used in qPCR were as follows: (Table 1)
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) |
|---|---|---|
| PSMC2 | CAGCACTCTGGGATTTGGCT | TTTCTATCCACGCCCACTCTC |
| GAPDH | TGACTTCAACAGCGACACCCA | CACCCTGTTGCTGTAGCCAAA |
Table 1: The sequences of primers in qPCR.
Western blot assay
The total proteins were extracted and quantified with BCA protein assay kit (Thermo Fisher Scientific, Cat. #A53227). After that, the proteins were segregated by 10% SDS-PAGE and transferred into PVDF membranes for Western blot assay. Then, the membranes were blocked and incubated with primary antibodies and second antibodies at room temperature for 2 h. Finally, the ECL+plusTM Western blotting system kit was used for color rendering and X-ray imaging was carried out. The primary antibodies used in western blotting were as follows: PSMC2 (1:2000, Mouse) and GAPDH (1:3000, Rabbit). The secondary antibody used in western blotting was Goat Anti-Mouse (1:3000, Beyotime, Beijing, China) and Goat Anti-Rabbit (1:3000, Beyotime, Beijing, China).
CCK8 assay
The MM.1R and MM.1S cells transfected with shPSMC2 and shCtrl were selected to digest, resuspend and count. 100 μL cell suspensions were added in a 96-well plate at the density of 3000 cell/well, and three replicates were set for each group. After that, the cells were placed in an incubator. From the second day, 10 μL CCK-8 was added into the well 2~4 h before the termination of the culture. After 4 h, the 96-well plate was placed on a shaker and oscillated for 2-5 min, and the OD value was measured for 5 days by the microplate reader at 450 nm. The experiment was repeated three times.
Transwell assay
Firstly, the upper chamber was incubated with 100 μL serum-free medium for 1-2 h. Then, the MM.1R and MM.1S cells transfected lentivirus were diluted and transferred into each chamber. At the same time, 600 μL medium with 30% FBS was added in the lower chamber.
After that, the upper chamber was transferred to the lower chamber and incubated for 40 h. 400 μL Giemsa were added for cells staining. Finally, the cells were dissolved in 10% acetic acid and the value of OD570 was detected. The experiment was repeated three times and the migration ability of cells was determined.
Detection of cell apoptosis and cell cycle by fluorescence activated Cells Sorting (FACS)
After infecting lentivirus, the MM.1R and MM.1S cells were inoculated in a 6-well plate (2 mL/well). When the cell confluence reached 85%, the cell suspension was centrifuged and the supernatants were discarded. Then, the cells were washed with 4 pre-cooled D-Hanks (pH=7.2~7.4). Next, 10 μL Annexin V-APC (eBioscience, San Diego, CA, USA) was added for staining in the dark. The cell apoptosis level was measured and the apoptotic rate was analyzed with FACSCalibur (BD Biosciences, San Jose, CA, USA). Considering cell cycle, the MM.1R and MM.1S cells were plated in 6-cm dishes (5 mL/well). The cell suspension was processed as above. Then the cells were washed with 4 pre-cooled PBS and ethanol and stained with solution PI. The changes of cell cycle were detected by FACSCalibur (BD Biosciences, San Jose, CA, USA). Each experiment was repeated 3 times.
Statistical analysis
All data were analyzed by GraphPad Prism 6 (San Diego, CA, USA) and data were presented as the mean ± SD. T-test was used to evaluate the statistical differences and the value of P less than 0.05 was considered to be significantly different.
Results
PSMC2 is abundantly expressed in multiple myeloma
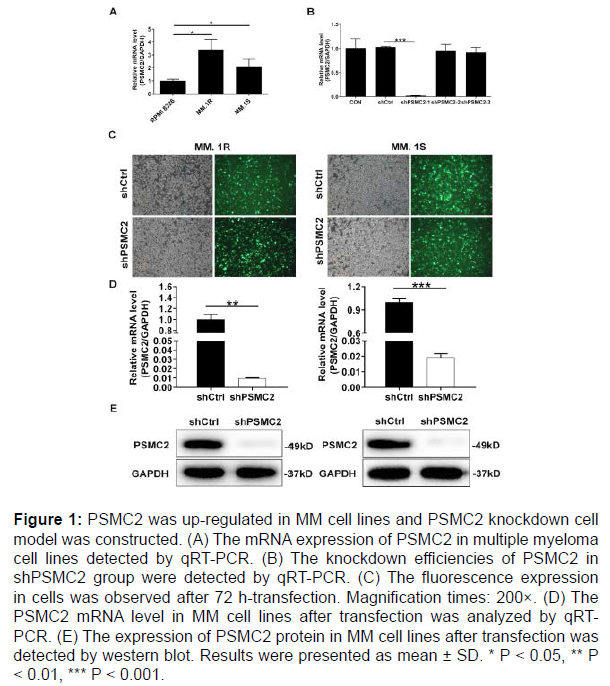
The expression of PSMC2 in multiple myeloma cell lines (MM.1R, MM.1S and RPMI 8226) was evaluated by qRT-PCR. As described in Figure 1A, the relative mRNA levels of PSMC2 were higher in both MM.1S and MM.1R cells compared with that in RPMI 8226 cells (P < 0.05). Thus, both MM.1S and MM.1R cells were selected for subsequent knockdown model experiments.
Construction of PSMC2 knockdown cell models
To reveal the roles of PSMC2 in multiple myeloma, the MM.1S and MM.1R cells models with PSMC2 knockdown were constructed. The effective interference targets were screened by qRT-PCR. It could be seen that the highest knockdown efficiency was observed in shPSMC2-1 group in MM.1S cells, reaching 98.1% (P < 0.001) (Figure 1B). At the same time, the green fluorescence, generated by the detection of the GFP inside the cells, was used to verify the successful transfection, which demonstrated > 80% transfection efficiencies in both MM.1R and MM.1S cells (Figure 1C). Moreover, the knockdown efficiency of PSMC2 was evaluated in both cells by qRT-PCR and western blot analysis. As appeared in Figure 1D, the knockdown efficiencies in shPSMC2 group were 99.0% (P < 0.01) and 98.1% (P < 0.001) in the MM.1R and MM.1S cells, respectively. Consistently, the results of western blot existed similar trends in shPSMC2 group of both cells, which revealed the down-regulated protein level (Figure 1E). The above results provided a reference for the successful establishment of PSMC2 knockdown cell models, which could be used in subsequent experiments.
Figure 1: PSMC2 was up-regulated in MM cell lines and PSMC2 knockdown cell model was constructed. (A) The mRNA expression of PSMC2 in multiple myeloma cell lines detected by qRT-PCR. (B) The knockdown efficiencies of PSMC2 in shPSMC2 group were detected by qRT-PCR. (C) The fluorescence expression in cells was observed after 72 h-transfection. Magnification times: 200×. (D) The PSMC2 mRNA level in MM cell lines after transfection was analyzed by qRTPCR. (E) The expression of PSMC2 protein in MM cell lines after transfection was detected by western blot. Results were presented as mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001.
Knockdown of PSMC2 inhibits proliferation of multiple myeloma cells
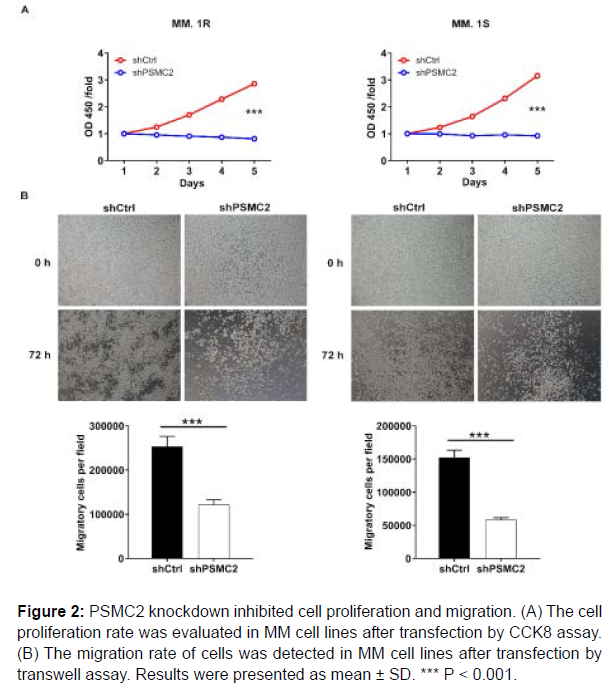
Subsequently, the effects of PSMC2 knockdown on proliferation of MM.1R and MM.1S cells were estimated by CCK8 assay. The results pointed out that the fold changes of the OD value were 3.5 and 3.4 in MM.1R and MM.1S cells, revealing that the cell proliferation rate exhibited significantly slower in shPSMC2 group in both cell lines (P < 0.001), (Figure 2A). These results denoted that PSMC2 knockdown could inhibit the proliferation of human multiple myeloma cells.
Knockdown of PSMC2 inhibits migration of multiple myeloma cells
In order to verify the effects of PSMC2 on metastasis, the transwell assay was conducted to assess the migration ability of human multiple myeloma cells. The results showed that the migration abilities of MM.1R and MM.1S cells in shPSMC2 group were decreased (P < 0.001), (Figure 2B). Therefore, it could be concluded that PSMC2 knockdown inhibited the migration of human multiple myeloma cells to a certain extent.
Figure 2: PSMC2 knockdown inhibited cell proliferation and migration. (A) The cell proliferation rate was evaluated in MM cell lines after transfection by CCK8 assay. (B) The migration rate of cells was detected in MM cell lines after transfection by transwell assay. Results were presented as mean ± SD. *** P < 0.001.
Knockdown of PSMC2 induces apoptosis and arrests cell cycle of multiple myeloma cells
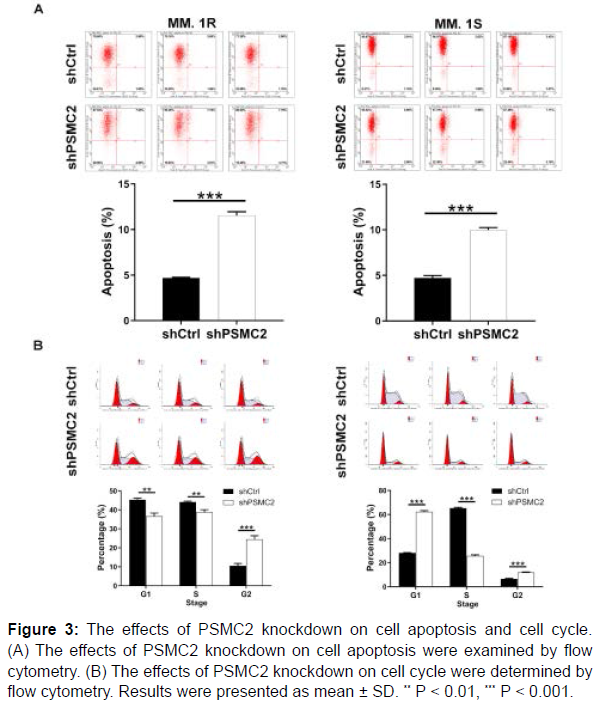
After the transfection of lentivirus, the flow cytometry analysis was performed to examine the effects of PSMC2 knockdown on cell apoptosis and cell cycle. As observed in Figure 3A, PSMC2 knockdown promoted the cell apoptosis of MM.1R and MM.1S cells (P < 0.001). On the other hand, the cell cycle analysis exhibited an increased percentage in G2 phase in shPSMC2 group in both cells (P < 0.001), (Figure 3B). In general, all above data confirmed that the knockdown of PSMC2 could promote apoptosis and disrupt cell cycle of human multiple myeloma cells.
Figure 3: The effects of PSMC2 knockdown on cell apoptosis and cell cycle. (A) The effects of PSMC2 knockdown on cell apoptosis were examined by flow cytometry. (B) The effects of PSMC2 knockdown on cell cycle were determined by flow cytometry. Results were presented as mean ± SD. ** P < 0.01, *** P < 0.001.
Discussion
Multiple myeloma is the second most common hematologic malignancy worldwide, representing approximately 2% of all malignancies and about 10% of all hematologic malignancies, and its incidence is on the rise [26]. Since the introduction of proteasome inhibitors such as bortezomib, carfilzomib and ixazomib [13], immunomodulators such as thalidomide, lenalidomide and pomalidomide [10], and monoclonal antibodies such as daratumumab and elotuzumab [11], the rate of life span and survival of patients with MM has greatly increased, but MM remains an incurable cancer.
Regarding current knowledge, the quantity of the 26S proteasome is regulated by PSMC2 and the 26S proteasome was reported to affect an ATP-dependent proteolytic degradation of many proteins such as cyclin-dependent kinase inhibitors, transcription factors, cell cycle specific cyclins, ornithine decarboxylase and other pivotal regulatory cellular events [27-29]. Because of the direct or indirect regulation of these cellular proteins related to the cancer progression, proteasomes have also been used for targeted cancer therapy [30]. Much work so far has focused on the development of 20S and 26S proteasome inhibitors for the treatment of various cancers, such as bortezomib, carfilzomib and ixazomib for MM [15], as well as proteasome inhibitors based on metal complexes for breast cancer, prostate cancer, pancreatic cancer and so on [20]. Based on the inseparable connection between PSMC2 and the 26S proteasome, oncology researchers initiated a new branch of theory including PSMC2 and malignant tumors.
Prior studies have identified functional roles of PSMC2 in certain human cancers. For instance, Song et al. [24] found that PSMC2 was up-regulated in osteosarcoma and involved in cell proliferation, migration and apoptosis. Nijhawan et al. [31] described that the downregulation of PSMC2 inhibited ovarian cancer cells proliferation and first announced the efficacy of the inhibition of PSMC2 in the treatment of ovarian cancer. PSMC2 was further certified as a representative of viability-related genes in ovarian cancer cells. In addition, Qin et al. reported that PSMC2 was up-regulated in pancreatic cancer and involved in the progression of PC by promoting cancer cell proliferation and inhibiting apoptosis [25]. Furthermore, miR-630 could predict the prognosis of osteosarcoma by targeting PSMC2, and regulate cell proliferation, migration and invasion [32]. Therefore, it could be basically concluded that PSMC2 played an important role in the occurrence and development of tumors. Nevertheless, there is still a lack of more extensive and systematic studies on the functional roles of PSMC2 in multiple myeloma. Based on this point of view, we conducted an elaborate research to explore the relationship between PSMC2 and human multiple myeloma.
In this paper, we firstly examined the expression levels and functional roles of PSMC2 in multiple myeloma cells. PSMC2 was highly expressed in multiple myeloma cells. It was further confirmed that PSMC2 knockdown could effectively make the PSMC2 expression in the mRNA and protein levels decreased in human multiple myeloma cell lines. It was noteworthy that the knockdown of PSMC2 inhibited cell proliferation and migration, induced apoptosis and caused cell cycle arrest. However, two issues may remain to be considered. One was whether PSMC2 levels correlated with other biomarkers of cell turnover such as B2M. Antigen-presenting protein beta-2-microglobulin (B2M), as one of the biomarkers of cell turnover, is a component of the human leukocyte antigen (HLA) class I molecule [33]. Previous study quantified the numbers of myeloma cell surface B2M and revealed that myeloma cells express 3-fold more B2M in comparison with normal blood lymphocytes [34]. Besides, it has come to light that 19S proteasome subunit, Sug1, is responsible for the mRNA expression of HLA-DM and HLA-DOB in B cells, therefore as an involvement in the regulating antigen presentation [35]. More importantly, bortezomib was reported to effectively reduce HLA antibody production with the help of the activation of plasma cells, thereby exerting the benefits of proteasome inhibition [36]. Based on these, we speculated that a potential relationship existed between PSMC2 expression and B2M, which required us to further verify. Another was whether knockdown of PSMC2 contributed anything beyond what was achieved with proteasome inhibitors. Qin et al. mentioned in a study investigating the association between PSMC2 and pancreatic cancer that excessive PSMC2 in normal cells resides a complex with PSMC1, PSMD2 and PSMD5, which plays a role in protecting cells from the antiproliferation caused by PSMC2 suppression. On the other hand, cells carrying part of the loss of PSMC2 lack this complex and die upon PSMC2 inhibition [31]. In conclusion, PSMC2 knockdown could inhibit multiple myeloma cell proliferation and enhance apoptosis and targeting PSMC2 might be an attractive target for novel therapeutic strategies. The in-depth regulation mechanism of PSMC2 on MM development needed to be further explored, which would be a focus of our subsequent attention.
Ethics approval
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Conflict of interests
The author declares that they have no conflict of interest.
Funding
No.
Contributors’ statement
All work was accomplished by Yuquan Ma.
References
- Ishida T, Kimura H, Ozaki S, Kubo K, Shimizu K (2020) Multiple Myeloma. Japanese J Clin Hematol 54(5): 89-99.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424.
- Kumar V, Sher T, Bojanini L, Vishnu P, Ailawadhi S, et al. (2018) Timeliness of Initial Therapy in Multiple Myeloma (MM): Trends and Factors Influencing Patient Care. Blood 132: 4764.
- Pulte ED, Dmytrijuk A, Nie L, Goldberg KB, Mckee AE, et al. (2018) FDA Approval Summary: Lenalidomide as Maintenance Therapy After Autologous Stem Cell Transplant in Newly Diagnosed Multiple Myeloma. Oncologist 23(6): 734-739.
- Nadeem O, Ghobrial IM (2020) Intensification and consolidation therapy in multiple myeloma in the current era. Lancet Haematol 7(6): e427-e429.
- Li JL, Fan GY, Liu YJ, Zeng ZH, Huang JJ, et al. (2018) Long-Term Efficacy of Maintenance Therapy for Multiple Myeloma: A Quantitative Synthesis of 22 Randomized Controlled Trials. Front Pharmacol 9: 430.
- Touzeau C, Moreau P (2018) Daratumumab for the treatment of multiple myeloma. Expert Opinion on Biological Therapy. Expert Opin Biol Ther 17(7): 887-893.
- Premkumar V, Bhutani D, Lentzsch S (2020) Modern Treatments and Future Directions for Relapsed/Refractory Multiple Myeloma Patients. Clin Lymphoma Myeloma Leuk 20(11): 736-743.
- Claudio C, Davide N, Maria D, Irene Z, Ilaria M, et al. (2017) A case of efficacy of bendamustine in heavily pretreated multiple myeloma, refractory to pomalidomide. Clin Case Rep 5(4): 505-507.
- Fernández-Lázaro D, Fernández-Lázaro CI (2018) Immunomodulator drugs for the treatment of multiple myeloma. Rev Med Chil 146(12): 1444-1451.
- Varga C, Maglio M, Ghobrial IM, Richardson PG (2018) Current use of monoclonal antibodies in the treatment of multiple myeloma. Br J Haematol 181(4): 447-459.
- Khan SB, Maududi T, Barton K, Ayers J, Alkan S (2015) Analysis of histone deacetylase inhibitor, depsipeptide (FR901228), effect on multiple myeloma. Br J Haematol 125(2): 156-161.
- Acostaalvear D, Cho MY, Wild T, Buchholz TJ, Lerner AG, et al. (2015) Paradoxical resistance of multiple myeloma to proteasome inhibitors by decreased levels of 19S proteasomal subunits. eLife 4: e08153.
- Sherman DJ, Li J (2020) Proteasome Inhibitors: Harnessing Proteostasis to Combat Disease. Molecules 25(3): 671.
- Perfetti V, Palladini G, Brunetti L, Sgarella A, Brugnatelli S, et al. (2007) Bortezomib-induced paralytic ileus is a potential gastrointestinal side effect of this first-in-class anticancer proteasome inhibitor. Eur J Gastroenterol Hepatol 19(7): 599-601.
- Ciehanover A, Hod Y, Hershko A (1978) A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun 81(4): 1100-1105.
- https://rgd.mcw.edu/rgdweb/report/gene/main.html?id=3428
- Adams J (2004) The proteasome: a suitable antineoplastic target. Nat Rev Cancer 4(5): 349-60.
- Groll M, Heinemeyer W, Jäger S, Ullrich T, Bochtler M, et al. (1999) The catalytic sites of 20S proteasomes and their role in subunit maturation: A mutational and crystallographic study. Proc Natl Acad Sci USA 96(20): 10976-10983.
- Shagufta, Ahmad I (2020) Transition metal complexes as proteasome inhibitors for cancer treatment. Inorg Chim Acta 506: 119521.
- Tanahashi N, Suzuki M, Fujiwara T, Takahashi EI, Shimbara N, et al. (1998) Chromosomal localization and immunological analysis of a family of human 26S proteasomal ATPases. Biochem Biophys Res Commun 243(1): 229-232.
- Yanagi S, Shimbara N, Tamura TA (2000) Tissue and Cell Distribution of a Mammalian Proteasomal ATPase, MSS1, and Its Complex Formation with the Basal Transcription Factors. Biochem Biophys Res Commun 279(2): 568-573.
- Kaneko T, Hamazaki J, Iemura SI, Sasaki K, Furuyama K, et al. (2009) Assembly Pathway of the Mammalian Proteasome Base Subcomplex Is Mediated by Multiple Specific Chaperones. Cell 137(5): 914-925.
- Song M, Wang Y, Zhang Z, Wang S (2017) PSMC2 is up-regulated in osteosarcoma and regulates osteosarcoma cell proliferation, apoptosis and migration. Oncotarget 8(1): 933.
- Qin J, Wang W, An F, Huang W, Ding J (2019) PSMC2 is Up-regulated in Pancreatic Cancer and Promotes Cancer Cell Proliferation and Inhibits Apoptosis. J Cancer 10(20): 4939-4946.
- Vincent RS (2018) Multiple myeloma: 2018 update on diagnosis, risk: tratification, and management. Am J Hematol 93(8): 1091-110.
- Deshaies, Raymond J (2014) Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol 12(1): 94.
- Liu N, Liu C, Li X, Liao S, Song W, et al. (2014) A novel proteasome inhibitor suppresses tumor growth via targeting both 19S proteasome deubiquitinases and 20S proteolytic peptidases. Sci Rep 4: 5240.
- Zhu K, Dunner K, Mcconkey DJ (2009) Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene 29(3): 451-462.
- Ellison-Zelski SJ, Casa AJ, Lee AV, Alarid ET, Powers GL (2009) Proteasome inhibition represses ERα gene expression in ER+ cells: a new link between proteasome activity and estrogen signaling in breast cancer. 29(10): 1509-1518.
- Nijhawan D, Zack TI, Ren Y, Strickland MR, Lamothe R, Schumacher SE, et al. (2012) Cancer Vulnerabilities Unveiled by Genomic Loss. Cell 150(4): 842-854.
- Li GW, Yan X (2019) Lower miR-630 expression predicts poor prognosis of osteosarcoma and promotes cell proliferation, migration and invasion by targeting PSMC2. Eur Rev Med Pharmacol Sci 23(5): 1915-1925.
- Zhang Q, Liu W, Xu H, Huang Z, Luo N, et al. (2020) 121P Relationship between different mutation type in JAK1/2/3 and B2M with other biomarkers for immunotherapy in solid tumours. Ann Onc 31: S288-S289.
- Yang J, Cao Y, Hong S, Li HY, Kwak LW, et al. (2009) B012 Efficacy and Safety of Anti-b2M mAbs to Treat MM. Clin Lymphoma Myeloma 9: 96.
- Inostroza-Nieves Y, Venkatraman P, Zavala-Ruiz Z (2012) Role of Sug1, a 19S proteasome ATPase, in the transcription of MHC I and the atypical MHC II molecules, HLA-DM and HLA-DO. Immunol Lett 147(1): 67-74.
- Guthoff M, Schmid-Horch B, Weisel KC, Haring HU, Konigsrainer A, et al. (2012) Proteasome inhibition by bortezomib: Effect on HLA-antibody levels and specificity in sensitized patients awaiting renal allograft transplantation. Transpl Immunol 26(4): 171-175.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ma Y (2022) PSMC2 Knockdown Inhibits Multiple Myeloma Cell Proliferation and Enhances Apoptosis. Cell Mol Biol, 68: 226. DOI: 10.4172/1165-158X.1000226
Copyright: © 2022 Ma Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1625
- [From(publication date): 0-2022 - Dec 06, 2023]
- Breakdown by view type
- HTML page views: 1248
- PDF downloads: 377