Recent Advances and Applications of Biosensors in Novel Technology
Received: 27-Jun-2017 / Accepted Date: 24-Aug-2017 / Published Date: 30-Aug-2017 DOI: 10.4172/2090-4967.1000145
Abstract
In recent time, several novel biosensors such as enzyme based, immunosensors, tissue-based, DNA biosensors, piezoelectric, and thermal biosensors have been explored with high sensitivity by many research groups. These biosensors exhibit many essential benefits, including operational simplicity, remarkable sensitivity, low-cost instrumentation, the potential of automation, inherent miniaturization. They have offered elegant paths for the detection of even trace amounts of analytical agents of biological significance existing from macromolecules (e.g., disease biomarkers and antigens) and small molecules (e.g., natural toxins and haptens) to cells, viruses, and bacteria. Many electrochemical techniques viz., amperometry and voltammetry, photoelectrochemistry, electrochemiluminescence, impedance, piezoelectricity, potentiometry, alternating current electrohydrodynamics, and field-effect transistor have been utilized in the development of immunoassays to attain high sensitivity for electrochemical signal transduction. Recent developments in biological methods and instrumentation after using fluorescence tag to various nanocarriers such as nanoparticles, nanowires, nanotubes, etc. have improved the sensitivity of biosensors. Utilization of nucleotides/aptamers, affibodies, molecule imprinted polymers, and peptide arrays offer great tools to prepare advanced biosensors. Merging of nanotechnology with biosensor systems enhanced the diagnostic capability. This review gives an outline of the advances in biosensors technology relating biomedical sciences.
Keywords: Biosensors; Nanomaterials; Electrochemical immunosensor; Optical biosensors; Surface plasmon resonance; Glucose biosensors; Bioreceptors
Introduction
The biosensor can be defined as a device that utilizes biological molecules or living organisms such as antibodies and enzymes to identify chemicals. The biosensor was first introduced in the 1960s and described the application of enzyme based bioelectrodes and their biocatalytic action. Afterward, in 1962, Clark presented a biosensor based model involving an oxygen electrode for the detection of electrochemical such as hydrogen peroxide or oxygen for the bioanalytical application. Later, the use of biosensors has expanded in the frontier of interdisciplinary research, i.e., analytical chemistry, bioelectronics, combines biology, and physics [1]. Different types of biosensors are being employed, which includes enzyme based, immunosensors, tissue-based, DNA biosensors, piezoelectric, and thermal biosensors [2].
The biosensor is a potential analytical device, which is used as a biological element (e.g., cell receptors, tissue, organelles, enzymes, microorganisms, nucleic acids, antibodies, etc.) to interacts (i.e., recognizes or binds) with an analyte [3]. A biosensor usually consists of three elements; a biorecognition, biotransducer, and an electronic system that include a processor, signal amplifier, and display [4,5]. The recognition part often termed as a bioreceptor, which exploits biomolecules as receptors to interact with a specific analyte. High selectivity of the analyte with bioreceptor is necessary. Biosensors are classified according to their types of interactions between biosensor and analyte, i.e., antibody/antigen, nucleic acids/DNA, enzymes/ ligands, biomimetic materials or cellular structures/cells [6].
Biosensors exhibit widespread applications in numerous field viz ., in novel drug discovery, biomedicine, diagnosis, food safety and processing, environmental monitoring, security, and defense. Pharmaceutical residues have conventionally been detected via qualitative/semi-quantitative screening techniques. Earlier, only distrusted samples were analyzed for validation by chromatographic techniques, i.e., high-performance liquid chromatography (HPLC) or gas chromatography (GC) attached to mass spectrometry (MS). Afterward, most of the pharmaceutical molecules have been derivatized to achieve good volatility for testing via gas chromatography. Nowadays HPLC-MS has shown wide acceptance (e.g., estrogens and antibiotics) in the analysis. For instance, capillary electrophoresis (CE) is another technique, which has not been accepted widely for analysis owing to its lack sensitivity over HPLC. Current advances in sample-preparation methods, detectors, and other apparatuses have enhanced the limits of detection (LODs). Instead, immunochemical methods with high-sample throughput ability could be used to achieve demands of pharmaceutical examinations [7].
The miniaturization of electrochemical immunosensors on microfluidic stages can not only overcome the limitations of conventional methods, including long detection times and the use of large volumes of reagents but also expand the use of immunosensors as a point of care tools [8-10].
Types of Bioreceptors
Enzymatic interactions
The enzyme-based sensor was first-time described in 1967 by Updike and Hicks [11]. In enzymatic interactions, initially, the substrates molecules, which is specific to an enzyme, bind to the enzyme and converts this complex into products. The mostly used bioreceptor include glucose oxidase (GOD), polyphenol oxidase (PPO), and urease. The enzymatic interactions exhibit some advantages such as; are highly selective, show catalytic activity, which improving sensitivity, and are fast acting. Some limitations are also associated with this are expensive (i.e., high cost of the source, extracting, isolating, and purifying), the activity might be lost during immobilization on a transducer, loss of activity owing to deactivation after a short period of time (due to storage in non-appropriate conditions).
Catalytic activity and explicit binding of enzyme make them potential bioreceptors. Analyte identification is enabled via several mechanisms: a) enzyme activation/inhibition is detected via the analyte; b) conversion of the analyte to a final product by the enzyme, which is detected by the sensor, or c) modifications in enzyme properties, which are occurring due to enzyme-analyte interaction is monitored [12]. The use of enzymes as a biosensor has some benefits such as: b) they can recognize a wide range of analytes (substrates, modulators, products, and inhibitors); a) they have the capability to catalyze several of reactions; and c) they are suitable for different transduction approaches to detect the analyte. Especially, enzymes are not used up in reactions. Hence biosensor can be utilized unceasingly. Though, biosensors have limited lifespan owing to limited enzymes stability.
The tyrosinase (Tyr), as an immunoassay label, can convert a weakly electroactive phenol to a highly electroactive catechol to trigger a β- nicotinamide adenine dinucleotide disodium salt (NADH)-related catalysis, which leads to a high signal-to-noise ratio for the immunoassay [13]. To minimize electrochemical signal interference, Yang and coworkers designed a washing-free electrochemical immunosensor with glycerol-3-phosphate dehydrogenase (GPDH) as an enzyme label. Because the reaction of GPDH with dissolved oxygen is low and the electrochemical interference of Ru(NH3)6 2+, an electrochemical oxidation product generated by GPDH, is also very low [14]. Xu et al. developed a highly sensitive electrochemical aptasensor by using nanocarriers of porous platinum nanotubes grafted with polyamidoamine based dendrimers as electrocatalysts for detection of thrombin. The enzyme-based signal amplification results showed a low detection limit, i.e., 0.03 pM [15].
Antibody/antigen interactions
Antibodies (Ab) or immunoglobulins proteins can bind to a foreign antigen (Ag) and assist in neutralizing foreign/invading particles.
Ab + Ag ↔ Ab∙Ag
Antibodies-antigens interaction is very selective and ultra-sensitive, they can bind with high power (Ka=106). Despite advantages, it presents some limitations also such as no catalytic effect is seen, only one-time use of biosensor is possible (disposable strips). Immunosensors, for instance, antibodies, utilize high binding affinity for a specific molecule or antigen. This specific property of antibodyantigen interaction is similar to the lock and key fit where antigen binds only to a specific antibody. This linking result into a physicochemical alteration in enzymes, radioisotopes, or fluorescent molecules, which produce a signal. Some limitations that include are; the antibody-antigen interaction is usually irreversible, and the binding capacity of an antibody is extremely dependent on conditions of the assay (e.g., temperature and pH). However, organic solvents, chaotropic reagents, and ultrasonic radiation can disrupt antibody/antigen interaction [12]. In an approach, a small protein with promising biophysical characteristics has been generated as antigen binding proteins with the specific binding ability to target various proteins without affecting properties of the molecule. Selection of elements, which can specifically attach to a target antigen, was performed (in vitro ) via phage display, yeast display, ribosome display, or mRNA display. However, artificial proteins for binding are usually smaller (i.e., <100 amino-acid residues) than antibodies. Нey have high stability with deficiency of disulfide bonds in contrast to antibodies [16,17]. Artificial proteins are thus a promising candidate to create biosensors [18,19]. Li et al. studied a non-enzymatic immunosensor as an important tool for the detection of carbohydrate antigen 15-3 using hierarchical nanoporous PtFe alloy [20,21].
Nucleic acid interactions
The nucleic acid is the building blocks of genetics. It operates selectively owing to base-pairing features, If the sequence of bases containing certain known part of the nucleic acid molecule, then the complementary sequence of it can be synthesized (called as a probe) and labeled using an optically detectable agent (eg., a fluorescent label). It has shown great potential in the detection of several types of cancers [22,23], viral [24] infections, and genetic [25] disorders. The nucleic acid biosensors are developed on the principle that the singlestrand of the nucleic acid molecule has the capability to identify and bind to its complementary strand. The interaction occurs owing to the formation of hydrogen bonds between the strands of two nucleic acids [26].
Biosensors that interact with nucleic acid are called as genosensors. In this type of interaction complementary base-pairing principle is used for recognition for example in DNA; adenine-thymine and cytosine-guanine. Complementary sequences are synthesized using known target nucleic acid sequence, labeled, and afterward immobilized on the sensor. Hybridization probes form a base pair with target sequences and thus generate optical signals. These optical signals are detected and favor transduction principle [12]. Song et al. induced DNA hybridization (in situ) via utilizing silver NPs aggregate as an electrochemical aptasensor (disposable) for signal amplification [27,28]. In another study, a gold nanomaterial and graphene based DNA biosensor showed high sensitivity [29]. Khimji et al. developed DNA-functionalized polyacrylamide hydrogels based optical biosensors [30]. Several DNA based biosensor utilizing NPs are being developed, which include, e.g., gold and magnetic nanoparticles [31], Pt nanoparticle [32], Pt nanoparticles/carbon nanotubes [33], and silver nanoparticles [28].
Cells and organelles
Cells are a cheaper source of enzymes as compared to many isolated enzymes, are less prone to inhibition via solutes and more stable at different temperature and pH condition that leads to extended lifetimes. Cells are often employed in bioreceptors since they are highly sensitive to surrounding atmosphere. They react to all types of stimulants. Cells are easily immobilized owing to their tendency to attach to the surface to facilitate immobilization. Cells can persist their activity for an extended period over organelles. They are usually utilized to monitor treatment results of drugs and can detect various parameters viz . toxicity, organic derivatives, and stress condition. Cells are employed to control herbicides, which are a main aquatic pollutant [34]. In another cell application, quartz microfiber is used to entrap micro algae, and then chlorophyll fluorescence is modified via the herbicides, which is collected from the tip of a bundle of the optical fiber and detected through a fluorimeter. Results reveal that certain herbicide detection limit can be reached up to sub-ppb concentration level. microbial corrosion is also monitored by using few cells [35]. Organelles inside a cell perform distinct functions independently. Organelles using specific enzymes carry out various essential metabolic pathways and fulfill body need. Some typical organelles include mitochondria, chloroplast, and lysosome. Mitochondria exhibits a substantial role in calcium ion metabolism to control functioning and modulating the calcium associated signaling pathways. It also presents some advantages such as sometimes it shows longer response times and recovery times, substrate requires to diffuse into the cytoplasm, and have less selectivity (as demonstrated by tissues owing to many enzymes). Elsewhere results revealed that mitochondria hold potential to respond high concentration of calcium that generates inside the proximity owing the opening of the calcium channel [36]. Thus, mitochondria are utilized to detect calcium concentration with high sensitivity (e.g., in water pollution detection) [37].
Tissues are employed as a biosensor for the plenty of enzymes. Some important advantages of tissues as biosensor include [38]: a) tissues can be immobilized easily as compared to organelles or cells, b) essential cofactors already exist for well-functioning of enzyme, c) Low cost and easy availability, d) the extraction, centrifugation, and purification of enzymes is not essential, e) a wide range of possibilities are available to fulfil different objectives, and f) in natural environment, enzymes show higher activity and stability. Despite advantages, tissues show some disadvantages such as they show less selectivity as compared to purified enzymes since they contain a variety of enzymes, reduced substrate specificity, slower response time, require more tissue material as a substrate to diffuse through, and this decrease the effect of enzymes.
Epigenetics
The term epigenetics in its contemporary application emerged in the 1990s; Epigenetic is the stably heritable phenotype resulting from variations in an organism that is caused due to modification of gene expression instead of a change in the genetic code itself. Photonic biosensors have been developed, which can detect tumor cell in a urine sample to an ultra-sensitivity level [39]. Elsewhere, epigenetic modifications are detected after exploitation of integrated optical resonators (e.g., post-translational modifications in histone and DNA methylation) using body fluids of patients suffering from cancer or other ailments [40].
Surface Attachment of Biological Elements
A vital step in relating to biosensor is linking of biological elements (i.e., cells/small molecules/proteins) on the surface of the sensor (e.g., polymer, glass, or metal,). However, the simplest method of the surface attachment is the functionalization of the surface via coating with the biological element. This is achieved by epoxysilane, aminosilane, nitrocellulose, or polylysine. Afterward, the attached biological molecules can be fixed via Layer-by-layer deposition using coatings of charged polymer [41]. Instead, 3-dimensional lattices (xerogel/hydrogel) can be employed to entrap these biological agents by chemical/physical method. The commonly utilized hydrogel is glassy silica (i.e., sol-gel), which is developed after polymerization of monomers of a silicate such as- tetra alkyl orthosilicates (e.g., TMOS/TEOS) in the presence of biological elements and PEG [42].
Placement of biosensors
The suitable positioning of biosensors is decided to owe their field of applications viz . biotechnology, biomedicine, food technology, and agriculture. In biomedical applications, biosensors are usually categorized according to in vitro or in vivo application. in vitro biosensor investigations are performed inside a test tube, a microtiter plate, a culture dish, or somewhere else outside the living animal. The sensor includes a bioreceptor and a transducer. Enzyme conductimetric based biosensor is a good example of in vitro type of biosensor utilized for determination of blood glucose level [43]. The exclusion of lab examination can save both time and money. However, POCT biosensor is utilized for confirming HIV virus, where it is not possible to test patients. Now it is easier to perform quick and easy testing using a biosensor. Whereas, a biosensor for in vivo is a device, which is implanted within the body. Hence, in vivo biosensor used for implants must be sterilized fulfill all the strict regulations to avoid primary inflammatory response [44].
Types of Biotransducer
Biosensors are classified as: a) electrochemical biosensors, b) optical biosensors, c) electronic biosensors, d) piezoelectric biosensors, e) gravimetric biosensors, and f) pyroelectric biosensors. Few important biosensors are described here.
Electrochemical
Electrochemical biosensors usually worked on the principle of enzymatic catalysis that generates or uses electrons (i.e., redox enzymes). Sensor substrate generally includes three electrodes; a) a reference electrode, b) a working electrode, and c) a counter electrode. The target analyte, which is used found on the electrode active surface, and the reaction may result in either transfer of electron across the double layer or can produce a potential to the double layer. The flow rate of electrons is directly proportional to the concentration of the analyte [45]. Potentiometric biosensor produces a logarithmic response. These biosensors are prepared using screen printing of electrode patterns over the surface of the plastic substrate and coated using the conducting polymer attached to some enzyme or antibody. All biosensors generally require very less quantity of sample since biological sensors are highly selective for the analyte. The signal is generated owing to physical and electrochemical variations in the layer of conducting polymer owing to changes over the surface of the sensor. These variations can be ascribed to pH, redox reactions, ionic strength, and hydration. Wang et al. studied the role of non-mediating ultrasensitive electrochemical immunosensors in Au/Ag/Au core/double shell NPs as enzyme-mimetic labels [46].
Electrochemical immunosensor
Many electrochemical immunosensor utilizing novel carrier systems have been developed for instance; carbon nanotube/manganese dioxide [47], graphene and Cu@Ag core-shell NPs [48], gold NPs [49]. Horseradish peroxidase and ferroferric oxide were used as a signal amplification labels in electrochemical immunosensor for detection of alpha-fetoprotein [50]. In milk, magneto immunosensor was also employed as electrochemical immunosensor for the detection of fluoroquinolone antibiotics [51]. Akter et al. reported enhancement in sensitivity of an electrochemical immunosensor via the electrocatalysis method in magnetic bead-supported non-enzymatic labels [52]. In another investigation, a simultaneous triple signal amplification effect was studied using bi-enzyme, gold NPs, and platinum NPs functionalized graphene as enhancers for multiple electrochemical immunoassays [53]. Recently, Reverte et al. reviewed the application of electrochemical biosensors in the detection of toxins using magnetic beads, microfluidics systems, and nanomaterials [54]. In another review, Wen et al. highlighted the recent progress of electrochemical immunosensors in conventional electrochemical methodologies of amperometry and voltammetry, electrochemiluminescence, impedance and photoelectrochemistry, and electroanalytical platforms and emerging devices [13].
Impedimetric biosensors
Impedimetric devices permit direct recognition of biomolecular activities without utilizing enzyme. In the biomedical investigation, impedimetric biosensors are widely employed in the label-free recognition of the ciprofloxacin (an antibiotic drug) at a very low concentration, (3 pmol/L) [55]. In a study, Du et al. prepared an impedimetric aptasensor for detection of the cocaine in the urine, saliva, and plasma of human [56].
Amperometric biosensors
Conventionally, amperometric biosensors used were dependent on the signal produced by labeled enzymes, which yielded electroactive components as a product of an enzymatic reaction [57-59]. Some molecules such as; penicillin [60], phenolic compounds and synthetic estrogens [61], anabolic androgenic steroids [62], and xenoestrogens [63] have been investigated via amperometric immunosensors.
Nanostructure based transducer surfaces have attracted in the recent progress of biosensors. These biosensors offer enhanced selectivity and sensitivity over existing techniques. Kim and coworkers prepared a chloramphenicol containing amperometric immunosensor using cadmium sulfide nanoparticles and modified using dendrimer bonded to the conducting polymer (AuNP) [64]. An amperometric immunosensor (label-free) utilizing Ag-NPs decorated with infinite coordination polymer fibers for carcinoembryonic antigen has been reported [57]. Hayat et al. developed a label-free novel amperometric immunosensor for the detection of okadaic acid [58]. Okadaic acid was also detected using amperometric immunosensor with great sensitivity in mussel sample via automated flow-through method [59]. In another study, a selective ultrasensitive detection of cortisol (metabolic regulator hormone) was developed after functionalizing gold nanowires and was aligned in such a way to work as an electrode in a three-electrode system [65]. Elsewhere, one of sub-unit was assembled using a gold support and the other on catalytic platinum NPs (PtNPs). Afterward, the creation of cocaine-aptamer complex was confirmed via PtNP-mediated reduction of hydrogen peroxide in the presence of cocaine. AuNP-grafted carbon nanotubes with various hydrazine labels have been utilized in an amplification approach to detect neomycin (an antibiotic) with a limit of detection (LOD) of 6.76 ± 0.17 ng/mL [66].
Ion channel switch
Ion channels offer high sensitivity in detection of biological molecules [67]. It is utilized for the quantitative detection of a wide range of target molecules, especially drug, proteins, toxins, and bacteria [68]. Ion channels are embedded in a tethered bilayer membrane and linked on the gold electrode, which generates an electric circuit. The capture biomolecules like antibodies are bound to the ion channel in such a way that the bonded target molecule controls the flow of ions through a channel. This produces a significant variation in the electrical conduction that is directly proportional to concentration. Biosensor based on ion channel can be formed using gramicidin (a chain of the dimmer peptide), inside the tethered bilayer membrane [69]. An antibody linked one of the peptides of the gramicidin is mobile, and other is static. The flow of ionic current stops through the membrane when dimer is broken. However, the extent of the variation in electrical signal is significantly amplified after separating of the membrane from the metal surface via a hydrophilic spacer.
Optical biosensors
First optical biosensor for commercial purpose was introduced in the late 1980s, since then a large number of optical biosensors has been described in research and development especially in diagnostic and pharmaceutical industries. These researches include virology [70], epitope mapping [71], ligand fishing [72], cell biology [73], cell adhesion [74], bacteriology [75], nucleotide–nucleotide [76] binding, enzyme mechanisms [77,78], molecular engineering [79], nucleotide–protein [30,80], and signal transduction [81,82].
Optical biosensors offer some great advantages as compared to conventional analytical methods since they enable real-time, label-free, and direct detection of numerous chemical and biological substances. Their advantages include sensitivity, high specificity, cost-effectiveness, and small size [83]. Optical biosensors can develop a cheap, multiplexed, and easily portable systems. Despite all these advantages, it can carry out label-free measurements. Newly fabricated nanostructures with size in nanometers have potential to direct light and can be utilized to examine different optical properties and phenomena of materials [84].
Microarrays
Microarrays are bi-dimensional receptor arrays, which offer simultaneous identification of several substances. Microarrays also have potential to detect small sized molecules, especially in biomedical. Peng et al. [85] prepared microarray to analyze three veterinary active molecules residues (i.e., tylosin, chloramphenicol, and clenbuterol. This microarray was synthesized using an indirect format, where small molecules are immobilized on the surface of a glass slide to compete with corresponding drug-antibodies. Subsequent the antigen-antibody linking, outcomes were measured via a secondary antibody labeled using Cy5. The detectability was attained in terms of IC50 for drugs; tylosin, chloramphenicol, and clenbuterol as 10.53 mg/L, 0.14 mg/L, and 0.53 mg/L, respectively.
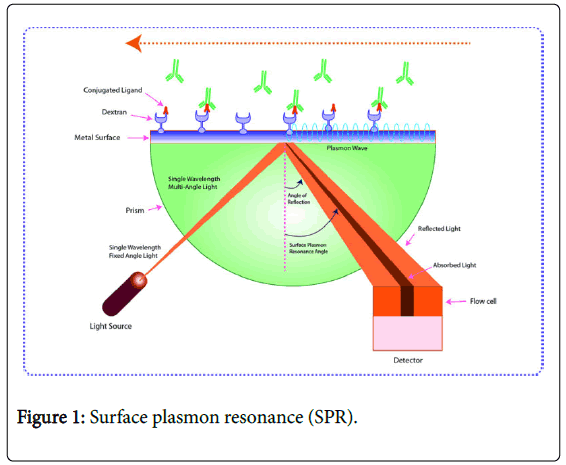
Surface plasmon resonance
Surface plasmon resonance (SPR) can be defined as the oscillation (resonant) of conduction electrons over the interface between positive and negative permittivity material stimulated via incident light. SPR method has been developed and exploited as a powerful label-free technique for the investigation of nucleic acid interactions. Surface plasmons or surface plasmon polaritons are produced via coupling between electrical field and free electrons present in a metal [86]. SPR waves circulate along the interface of a conducting layer, which is rich in free electrons and dielectrics. This method offers a simultaneous characterization of biomolecular interactions with high sensitivity using low quantity of sample. SPR technology has great application in protein-DNA interaction studies using biosensor-SPR approach [27].
Surface plasmons are widely used to increase the sensitivity of surface for several spectroscopic analyses including Raman scattering, second harmonic generation, and fluorescence (Figure 1). SPR) is being exploited in different areas, especially in pharmaceuticals [60]. Recently, Fernandez et al. synthesized an SPR biosensor for identification of antibiotics in the milk using multiplexed analysis [87]. This multiplexed portable biosensor containing six channels were employed to analyze three antibiotics of different families viz ., chloramphenicol, sulfonamides, and fluoroquinolones. The biosensor has been employed for detection of antibodies with wide-ranging spectrum. LODs values showed 1.1 μg/kg, 1.7 μg/kg, and 2.1 μg/kg for chloramphenicol, sulfapyridine, and enrofloxacin, respectively. These values highlight possibility of determining antibiotic residues lower the maximum residue limits.
Reagentless fluorescent (RF) biosensor
An RF biosensor facilitates detection of an analyte in the complex biological blend without using any other reagent. Hence, RF biosensor can work continuously when it is immobilized using a solid support. When a fluorescent biosensor interacts with an analyte, it results in an alteration in fluorescence properties. An RF biosensor is prepared after assimilating a biological receptor and a solvatochromic fluorophore. Fluorophore transduces the detection process into a quantifiable optical signal. Usually, an extrinsic fluorophore is widely utilized for which emission properties differ from intrinsic fluorophores of proteins such as tyrosine and tryptophan. This offer immediate detection and measuring of an analyte in the biological mixture. Both, antibodies and antigen binding proteins (AgBP) can be targeted directly against any kind of antigen, which makes them promise module for RF biosensors. When the structure of the antigen-complex is known, then this approach of integrating into AgBP of the solvatochromic fluorophore is used to convert into an RF biosensor [18]. Studies have suggested that fluorophore with the tendency of not forming a covalent bond on the surface of bioreceptor can produce RF biosensors of high quality owing to enhance in background signal due to interaction with the binding pocket over the antigen surface [88].
Glucose biosensors
The personal glucose meter (GM) is a typical device for point of care testing in household situations as of its comfort of use and consistent quantitative results. Recently, the DNA sensors, which were attached with a general GM were developed for the efficient detection of several targets, e.g., protein biomarkers [89,90] and recreational drugs [91,92].
Valentini et al. developed a glucose biosensor using gold microelectrodes coated via Single-Walled Carbon Nanotubes (SWCNTs), by the Electrophoresis Deposition Process. This nanostructured biosensor was successfully utilized to layer a poly (pyrrole)/glucose oxidase film. A highly extended linear concentration (ranging from 4 mM to 100 mM) of biosensor offered the opportunity to determine glucose level from 0.560 mM to 12.0 mM, with a high detection limit of 50 μM (useful for hypo-glycemia disease) [93]. A glucose biosensor working on the principle of direct transfer of an electron from glucose oxidase (GOD) and self-assembled over the surface of the electrochemically reduced carboxyl graphene (ERCGr) altered glassy carbon electrode has been prepared. X-ray photoelectron spectroscopy study of ERCGr showed that most of the oxygen-bearing groups in the carboxyl graphene, e.g., epoxy/ether and hydroxyl groups were removed excluding carboxylic acid. The cyclic voltammetric study of the electrode demonstrated a pair of quasi-reversible and welldefined redox peaks (-0.467 V) with a distance between peaks of 49 mV, which revealed that the electron transfer has occurred in between the electrode and GOD. The glucose biosensor exhibited a linear effect on the concentration of glucose in the range from 2 mM to 18 mM (detection limit=0.02 Mm) [94,95].
Applications
Biosensors exhibit several promising applications in the biomedical sciences (Table 1). Some of the important applications are discussed in this review.
| Transduction | Biosensor type | Applications | References |
|---|---|---|---|
| Electrochemical | Acetylcholinesterase (AChE) inhibition biosensors | Pesticidal study | [96] |
| Hba1c biosensor | Determining glycated hemoglobin | [97] | |
| Piezoelectric biosensors | Detecting carbamate and organophosphate | [98] | |
| Uric acid biosensors | Diagnosis of various clinical abnormalities or illness (e.g., diagnosis of cardiovascular disease) | [99,100] | |
| Glucose oxidase electrode biosensors | Measuring of glucose in biological sample of diabetic patient | [101] | |
| Quartz–crystal biosensors | Detection of proteins at ultrahigh-sensitive level in liquids |
[102] | |
| Optical | Polyacrylamide based hydrogel biosensors | Immobilization of biomolecules | [30] |
| Silicon biosensor | For biosensing, bioimaging, and in cancer therapy | [103,104] | |
| Microfabricated biosensor | In novel drug delivery (e.g., in optical corrections) | [105] | |
| Fluorescence tagged/Genetically encoded biosensor | Investigation of various biological process and molecular systems in the cell | [27,106-108] | |
| Electrochemical or optical | Nanomaterials biosensors | For diagnosis and in drug delivery | [32,93,109-114] |
Table 1: List of biosensors with their applications.
Glucose monitoring
Commercially available glucose monitoring is dependent on amperometric sensing of glucose via means of glucose oxidase that oxidizes glucose to produce hydrogen peroxide. Then, hydrogen peroxide is detected via the electrode. In order to overcome limitation owing to amperometric sensors. Several research articles are existing into novel sensing techniques, e.g., fluorescent glucose biosensors [115]. Palanisamy et al. studied successful recognition of the direct electrochemistry of GOD on modified electrode of silver NPs (RGO/Ag) nanocomposite and reduced graphene oxide [116]. In another study, Tang et al. demonstrated antigen-antibody interaction via GOD conjugated hollow gold-silver microspheres (AuAgHSs) further coupled using Prussian blue NPs (an artificial catalase), on a graphene-based immunosensor [117].
DNA biosensors
DNA biosensors (i.e., genosensors) are being utilized theoretically in different area viz ., forensic science, medical diagnostics, agriculture, and in environmental clean-up. A significant advantage of DNA-based sensing devices is that it does not require any external monitoring. However, DNA biosensors are complex mini machines, which consist of micro lasers, sensing elements, and signal generator. In DNA, two strands are stick to each other via virtue of attraction due to chemical forces. When two strands match up at each nucleotide site, it produces a fluorescent signal (glow), which then transmitted to the signal generator. Genosensors have been employed for their intrinsic suitability and physicochemical stability to distinguish various organism strains. DNA hybridization occurs specifically on the surface of the physical transducer; this principle is used for genosensors based detection [118].
Microbial biosensors
Many microbial based biosensors (e.g., arsenic biosensor) have been developed by scientists using biological engineering. Bacteria are used for detection of pollutants in the samples. In order to detect arsenic, biologists utilize Ars operon [119].
Nanomaterials as biosensors
The nanomaterial hybrids developed after utilizing nanostructures (i.e., nanowires, nanoparticles, carbon nanotubes, nanorods, etc.) reveal combined characteristics of the specific nano materials [120]. A complex of silver and gold NPs have been developed to intensify the electrochemical signal in affinity-based sensors [121]. Other nanocomposites explored for immobilization study include Fe3O4- chitosan, magnetic nanoparticles-silica, Pt/carbon nanotubes, and PANI/PVA-AgNP [122-125]. However, composite nanomaterials and their hybrid have shown great attraction as electrochemical biosensors. These hybrid constructions can act as signal amplifiers, immobilization supports, and probe for generating an electrochemical signal. For instance, Iron-silica complexes have been utilized for immobilization of biomolecule owing to their improved biocompatibility [126]. ZnO and Pt can be used in the preparation of several classes of nanomaterials to enhance the efficiency of amperometric biosensors [127]. Attaching of NPs to the QCM results into the large surface area, variability in shape, and physicochemical malleability. However, optional linking of metals empowers for different properties such as a) change in responses, b) specificity and c) selectivity. This transducer’s features in a grouping with NPs increase surface area to volume ratios, which form it a perfect biosensor to primarily measure the malignancy and metastatic power of cancer cells. Atay’s et al. investigated the power of metastatic (in vitro ) breast cancer. Results revealed that it requires nearly 58 nm, Poly (2-hydroxyethyl methacrylate) NPs having a surface area of approximately 1899 m2g-1 for effective adsorption of the cells to QCM surface (Table 2) [128].
| Biosensor type | Nanomaterial | Applications | References |
|---|---|---|---|
| DNA biosensor | Silver NPs | Amplification of signals | [28] |
| Gold NPs and graphene | Immobilization support | [29] | |
| Platinum nanotubes modified with poly-amidoamine | Amplification of signals | [15] | |
| Enzymatic biosensor | Carboxygraphene | Enhance electrical properties | [94] |
| Graphene nanoplatelet–titanate nanotube composite | Immobilization support | [129] | |
| Graphene oxide and silver NPs |
Immobilization support | [116] | |
| iron oxide–chitosan nanocomposite | Immobilization support | [130] | |
| Hydrogen per oxide sensor | Graphene oxide (ERGO)–silver NPs | Enzyme impersonators | [131] |
| MnO2 | Enzyme impersonators | [132] | |
| Immunosensor | Ferroferric oxide NPs | Amplification of signals | [50] |
| Gold NPs | Amplification of signals | [133] | |
| Gold NPs/polyaniline nanofibers |
Immobilization support | [134] | |
| Graphene, platinum NPs |
Amplification of signals | [53] | |
| PtCo Alloy and graphene | Mediator/signal generating probe | [135] | |
| Magnetic beads | Signal generating probe | [52] | |
| Cu@Ag (Cu@Ag-CD) core–shell NPs |
Signal generating probe | [48] | |
| Myoglobin biosensor |
Nano gold/graphene | Enhance electrical properties | [136] |
Table 2: Applications of some nanomaterials based electrochemical biosensors.
Nanomaterials biosensors in cancer cell
In contrast to radiology imaging tests that direct some forms of energy (i.e., magnetic fields and X-rays, etc.) through the body to take interior images only. Biosensors can directly examine the malignant influence of the tumor. Both detector and biological elements utilize a small quantity of sample to test. Biosensors exist with compact design, rapid detection, rapid signals, high sensitivity, and high selectivity for the analyte. As compared to typical radiology imaging, biosensors have many benefits such as- a) it determines the extent of spread of cancer, b) checking of the effectiveness of treatment, c) prove more efficient (in cost, time, and productivity). Biological researchers have developed oncological biosensors for the treatment of breast cancer [137]. Another example of the biosensor is a transferrin quartz crystal microbalance (QCM). Atay et al. revealed selectivity and the specificity (in vitro) between QCM and MCF 7 cells, MDA-MB 231 breast cells, and starved MDA-MB 231 cells. They developed a technique of washing these various metastatic cells as compared to sensors to calculate mass shifts owing to different amounts of transferrin receptors. Results showed high selectivity for transferrin receptors since they are over-expressed in cancer cells. They revealed higher binding affinity to QCM [137].
Human epidermal growth factor receptor 2 (HER2 or ERBB2) is a significant biomarker found in breast cancer and was detected by Electrochemical impedance spectroscopy using a self-assembled monolayer of cysteine [138]. Lipoproteins [139], serum oncomarker CA125 [140], and tissue polypeptide antigen [141] were also sensitively detected via impedance, and impedimetric derived immunoassays in ovarian cancer patients for early diagnostics. In another study, Wu et al. confirmed an electrochemical visual platform for the efficient detection of cancer biomarkers by the integration of a closed bipolar electrode array and driving electrodes into a multichannel chip [142]. Shi et al. developed a bipolar electrode sensing platform, which coupled an electrochemiluminescence sensor with anodic dissolution for the sensitive detection of cancer cells [143]. In another study, the microfluidic immunoarray presented exceptional performance (within 36 min) in the instantaneous detection of the four types of prostate cancer protein biomarkers [144].
Food analysis
In the food industry, antibodies coated optics are generally utilized to detect food toxins and pathogens. Since fluorescence based optical measurement system highly amplifies the signal. Hence, fluorescence is used as a light in these biosensors. A wide range of ligand-binding and immune assays are performed for detection and investigation of small molecules. Water-soluble vitamins and drug residues viz ., β-agonists and sulfonamides have been prepared to utilize SPR based sensor systems, often revised from current ELISA or from another immunological assay. The biosensor is an efficient, attractive, and alternative method to various other techniques. Since it is reliable and responds quickly. It showed high potential in the food industry for monitoring quality and safety of food as well as in bioprocessing industries [145].
Ozone biosensors
Ozone biosensors are important since ozone filters harmful ultraviolet radiation. The finding of the hole in the ozone layer has become a matter of worry. How much ultraviolet (UV) light reaches the surface of the earth and how deeply it reaches into sea water. Ultraviolet radiation can penetrate sea and can produce harmful effects on marine organisms, especially floating microorganisms (plankton). Plankton is the basis of marine food chains and is supposed to affect earth's weather and temperature via maintaining a balance between oxygen and CO2 by photosynthesis. Karentz et al. have developed a simple technique for measuring intensity and UV penetration. A thin plastic bags was submerged to several depths holding particular strains of E. coli . The study revealed that E. coli were impotent to repair damage caused via ultraviolet radiation to their DNA. The bacterial "biosensors" showed persistent significant damage owing to UV light at depths of 10 m and regularly at 20 m and 30 m [146].
Discussion
The first biosensor was introduced in the 1960s and described the application of enzyme based bioelectrodes and their biocatalytic action. Afterward, several types of biosensors are being designed and utilized that include; cell or tissue-based, enzyme based, immunosensors, thermal and piezoelectric biosensors, and nucleic acid biosensors.
Enzyme based biosensors are being developed using immobilization techniques, i.e., covalent or ionic bonding and adsorption of enzymes via van der Waals forces by utilizing enzymes such as polyphenol oxidases, oxidoreductases, amino-oxidases, and peroxidases. Whereas, the tissues-based sensors were designed from animal and plant sources. In addition, the organelle-based sensors were developed by exploiting chloroplasts, membranes, microsomes, and mitochondria. Organelle-based biosensor reveals high stability but shows longer time for detection with decreased specificity. Antibodies based biosensors have more affinity towards particular antigens, viz ., the antibodies bind specifically to the toxins or pathogens, or interact with different components of the immune system of the host. The nucleic acid biosensors are developed on the fact that single-strand of the nucleic acid molecule could recognize and attach to its complementary strand. The interaction occurs owing to the formation of hydrogen bonds between the nucleic acid strands.
Piezoelectric biosensors such as the surface acoustic wave device and the quartz crystal microbalance are based on the determination of deviations in resonance frequency in the piezoelectric crystal owing to weight changes in the structure of the crystal. First optical biosensor for commercial purpose was introduced in the late 1980s, since then a large number of optical biosensors has been described in research and development especially in diagnostic and pharmaceutical industries. These research include virology, epitope mapping, ligand fishing, cell biology cell adhesion, bacteriology, nucleotide–nucleotide binding, enzyme mechanisms, molecular engineering, nucleotide–protein, and signal transduction. Optical biosensors comprise of several optical components to produce a light beam having a specific property.
SPR method has been developed and exploited as a powerful labelfree technique for the investigation of nucleic acid interactions. This method offers a simultaneous characterization of biomolecular interactions with high sensitivity using low quantity of sample. SPR technology has great application in protein-DNA interaction studies using biosensor-SPR approach. Surface plasmons are widely used to increase the sensitivity of surface for several spectroscopic analyses including Raman scattering, second harmonic generation, and fluorescence. The personal GM is a typical device for point of care testing in household situations as of its comfort of use and consistent quantitative results. Recently, the DNA sensors, which were attached with a general GM were developed for the efficient detection of several targets, e.g., protein biomarkers and recreational drugs.
Conclusion
Several newly developed techniques ranging from electrochemical, optical including fluorescence-based, and electromechanical are modern transducing methods, which are widely utilized in the development of biosensors. Different biosensors are now available in various fields viz ., biomedicine, novel drug discovery, environmental monitoring, food safety processing, security, and defense. Thus, the invention of various powerful accurate and sensitive biosensors of immense importance e.g., electrochemical immunosensors, surface plasmon resonance sensor, glucose biosensors, nucleic acid biosensors and so on has offered many benefits over existing methods (i.e., improved sensitivity, less time, high-throughput screening, the possibility of developing label-free detection methods, and real-time analysis). Recent developments in biological techniques and instrumentation after utilizing fluorescence tag to various nano carriers namely; NPs, nanowires, nanotubes, etc. have improved the sensitivity of biosensors. Utilization of nucleotides or aptamers, affibodies, molecule imprinted polymers, and peptide arrays offer great tools to prepare advanced biosensors as compared to traditional techniques. Merging of nanotechnology with biosensor systems can enhance the diagnostic capability.
References
- Gonzalez-Rodriguez J, Raveendran M (2015) Importance of biosensors. Biosensors J 4: e104.
- Mehrotra P (2016) Biosensors and their applications– A review. J Oral Biol Craniofac Res 6:153-159.
- Banica F-G (2012) Chemical sensors and biosensors: Fundamentals and applications. John Wiley & Sons
- Hierlemann A, Baltes H (2003) CMOS-based chemical microsensors. Analyst 128: 15-28
- Hierlemann A, Brand O, Hagleitner C, Baltes H (2003) Microfabrication techniques for chemical/biosensors. Proc IEEE 91: 839-863.
- Vo-Dinh T, Cullum B (2000) Biosensors and biochips: advances in biological and medical diagnostics. Fresenius J Anal Chem 366: 540-551.
- Adrian J, Font H, Diserens JM, Sanchez-Baeza F, Marco MP (2009) Generation of broad specificity antibodies for sulfonamide antibiotics and development of an enzyme-linked immunosorbent assay (ELISA) for the analysis of milk samples. J Agric Food Chem 57: 385-394.
- Xia Y, Si J, Li Z (2016) Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens Bioelectron 77: 774-789.
- Arduini F, Micheli L, Moscone D, Palleschi G, Piermarini S, et al. (2016) Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC, Trends Anal Chem 79: 114-126.
- Samiei E, Tabrizian M, Hoorfar M (2016) A review of digital microfluidics as portable platforms for lab-on a-chip applications. Lab on a Chip 16: 2376-2396.
- Marazuela D, Moreno-Bondi MC (2002) Fiber-optic biosensors-an overview. Anal Bioanal Chem 372: 664-682.
- Wen W, Yan X, Zhu C, Du D, Lin Y (2017) Recent advances in electrochemical immunosensors. Anal Chem 89: 138-156.
- Dutta G, Park S, Singh A, Seo J, Kim S, et al. (2015) Low-interference washing-free electrochemical immunosensor using glycerol-3-phosphate dehydrogenase as an enzyme label. Anal Chem 87: 3574-3578.
- Xu W, Wu Y, Yi H, Bai L, Chai Y, et al. (2014) Porous platinum nanotubes modified with dendrimers as nanocarriers and electrocatalysts for sensitive electrochemical aptasensors based on enzymatic signal amplification. Chem Commun (Camb) 50: 1451-1453.
- Jost C, Pluckthun A (2014) Engineered proteins with desired specificity: DARPins, other alternative scaffolds and bispecific IgGs. Curr Opin Struct Biol 27: 102-112.
- Skrlec K, Strukelj B, Berlec A (2015) Non-immunoglobulin scaffolds: A focus on their targets. Trends Biotechnol 33: 408-418.
- Brient-Litzler E, Pluckthun A, Bedouelle H (2010) Knowledge-based design of reagentless fluorescent biosensors from a designed ankyrin repeat protein. Protein Eng Des Sel 23: 229-241.
- Miranda FF, Brient-Litzler E, Zidane N, Pecorari F, Bedouelle H (2011) Reagentless fluorescent biosensors from artificial families of antigen binding proteins. Biosens Bioelectron 26: 4184-4190.
- Schultz JS, Mansouri S, Goldstein IJ (1982) Affinity sensor: A new technique for developing implantable sensors for glucose and other metabolites. Diabetes Care 5: 245-253.
- Li Y, Xu C, Li H, Wang H, Wu D, et al. (2014) Nonenzymatic immunosensor for detection of carbohydrate antigen 15-3 based on hierarchical nanoporous PtFe alloy. Biosens Bioelectron 56: 295-299.
- Sohrabi N, Valizadeh A, Farkhani SM, Akbarzadeh A (2016) Basics of DNA biosensors and cancer diagnosis. Artif Cells Nanomed Biotechnol 44: 654-663.
- Monroig PdC, Chen L, Zhang S, Calin GA (2015) Small molecule compounds targeting miRNAs for cancer therapy. Adv Drug Del Rev 81: 104-116.
- Donmez S, Arslan F, Arslan H (2015) A nucleic acid biosensor for detection of hepatitis C virus genotype 1a using poly (L-Glutamic Acid)-modified electrode. Appl Biochem Biotechnol 176: 1431-1444.
- Mandli J, Mohammadi H, Amine A (2017) Electrochemical DNA sandwich biosensor based on enzyme amplified microRNA-21 detection and gold nanoparticles. Bioelectrochemistry (Amsterdam, Netherlands). pp. 116: 17-23.
- Wang J (1998) DNA biosensors based on Peptide Nucleic Acid (PNA) recognition layers. A review. Biosens Bioelectron 13: 757-762.
- Wang S, Poon GM, Wilson WD (2015) Quantitative investigation of protein-nucleic acid interactions by biosensor surface plasmon resonance. Methods Mol Biol 1334: 313-332.
- Song W, Li H, Liang H, Qiang W, Xu D (2014) Disposable electrochemical aptasensor array by using in situ DNA hybridization inducing silver nanoparticles aggregate for signal amplification. Anal Chem 86: 2775-2783.
- Shi A, Wang J, Han X, Fang X, Zhang Y (2014) A sensitive electrochemical DNA biosensor based on gold nanomaterial and graphene amplified signal. Sensors Actuators B: Chem 200: 206-212.
- Khimji I, Kelly EY, Helwa Y, Hoang M, Liu J (2013) Visual optical biosensors based on DNA-functionalized polyacrylamide hydrogels. Methods 64: 292-298.
- Yang CH, Kuo LS, Chen PH, Yang CR, Tsai ZM (2012) Development of a multilayered polymeric DNA biosensor using radio frequency technology with gold and magnetic nanoparticles. Biosens Bioelectron 31: 349-356.
- Kwon SJ, Bard AJ (2012) DNA analysis by application of Pt nanoparticle electrochemical amplification with single label response. J Am Chem Soc 134: 10777-10779.
- Dong XY, Mi XN, Zhang L, Liang TM, Xu JJ, et al. (2012) DNAzyme-functionalized Pt nanoparticles/carbon nanotubes for amplified sandwich electrochemical DNA analysis. Biosens Bioelectron 38: 337-341.
- Vedrine C, Leclerc JC, Durrieu C, Tran-Minh C (2003) Optical whole-cell biosensor using Chlorella vulgaris designed for monitoring herbicides. Biosens Bioelectron 18: 457-463.
- Dubey RS, Upadhyay SN (2001) Microbial corrosion monitoring by an amperometric microbial biosensor developed using whole cell of Pseudomonas sp. Biosens Bioelectron 16: 995-1000.
- Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, et al. (1999) Mitochondria as biosensors of calcium microdomains. Cell Calcium 26: 193-199.
- Bragadin M, Manente S, Piazza R, Scutari G (2001) The mitochondria as biosensors for the monitoring of detergent compounds in solution. Anal Biochem 292: 305-307.
- Campas M, Carpentier R, Rouillon R (2008) Plant tissue-and photosynthesis-based biosensors. Biotechnol Adv 26: 370-378.
- Vollmer F, Yang L (2012) Label-free detection with high-Q microcavities: A review of biosensing mechanisms for integrated devices. Nanophotonics 1: 267-291.
- Donzella V, Crea F (2011) Optical biosensors to analyze novel biomarkers in oncology. J Biophotonics 4: 442-452.
- Pickup JC, Zhi ZL, Khan F, Saxl T, Birch DJ (2008) Nanomedicine and its potential in diabetes research and practice. Diabetes Metab Res Rev 24: 604-610.
- Gupta R, Chaudhury NK (2007) Entrapment of biomolecules in sol-gel matrix for applications in biosensors: Problems and future prospects. Biosens Bioelectron 22: 2387-2399
- Kling J (2006) Moving diagnostics from the bench to the bedside. Nat Biotech 24: 891-893.
- Kotanen CN, Moussy FG, Carrara S, Guiseppi-Elie A (2012) Implantable enzyme amperometric biosensors. Biosens Bioelectron 35: 14-26.
- Lud SQ, Nikolaides MG, Haase I, Fischer M, Bausch AR (2006) Field effect of screened charges: Electrical detection of peptides and proteins by a thin-film resistor. Chemphyschem 7: 379-384.
- Wang Y, Zhang Y, Su Y, Li F, Ma H, et al. (2014) Ultrasensitive non-mediator electrochemical immunosensors using Au/Ag/Au core/double shell nanoparticles as enzyme-mimetic labels. Talanta 124: 60-66.
- Tu MC, Chen HY, Wang Y, Moochhala SM, Alagappan P, et al. (2015) Immunosensor based on carbon nanotube/manganese dioxide electrochemical tags. Anal Chim Acta 853: 228-233.
- Gao J, Guo Z, Su F, Gao L, Pang X, et al. (2015) Ultrasensitive electrochemical immunoassay for CEA through host-guest interaction of beta-cyclodextrin functionalized graphene and [email protected] core-shell nanoparticles with adamantine-modified antibody. Biosens Bioelectron 63: 465-471.
- Omidfar K, Zarei H, Gholizadeh F, Larijani B (2012) A high-sensitivity electrochemical immunosensor based on mobile crystalline material-41-polyvinyl alcohol nanocomposite and colloidal gold nanoparticles. Anal Biochem 421: 649-656.
- Wang H, Li X, Mao K, Li Y, Du B, et al. (2014) Electrochemical immunosensor for alpha-fetoprotein detection using ferroferric oxide and horseradish peroxidase as signal amplification labels. Anal Biochem 465: 121-126.
- Pinacho DG, Sanchez-Baeza F, Pividori MI, Marco MP (2014) Electrochemical detection of fluoroquinolone antibiotics in milk using a magneto immunosensor. Sensors (Basel) 14: 15965-15980.
- Akter R, Kyun Rhee C, Rahman MA (2014) Sensitivity enhancement of an electrochemical immunosensor through the electrocatalysis of magnetic bead-supported non-enzymatic labels. Biosens Bioelectron 54: 351-357.
- Jia X, Chen X, Han J, Ma J, Ma Z (2014) Triple signal amplification using gold nanoparticles, bienzyme and platinum nanoparticles functionalized graphene as enhancers for simultaneous multiple electrochemical immunoassay. Biosens Bioelectron 53: 65-70.
- Reverte L, Prieto-Simon B, Campas M (2016) New advances in electrochemical biosensors for the detection of toxins: Nanomaterials, magnetic beads and microfluidics systems. A review. Anal Chim Acta 908: 8-21.
- Giroud F, Gorgy K, Gondran C, Cosnier S, Pinacho DG, et al. (2009) Impedimetric immunosensor based on a polypyrrole-antibiotic model film for the label-free picomolar detection of ciprofloxacin. Anal Chem 81: 8405-8409.
- Du Y, Chen C, Yin J, Li B, Zhou M, et al. (2010) Solid-state probe based electrochemical aptasensor for cocaine: a potentially convenient, sensitive, repeatable, and integrated sensing platform for drugs. Anal Chem 82: 1556-1563.
- Lu W, Cao X, Tao L, Ge J, Dong J, et al. (2014) A novel label-free amperometric immunosensor for carcinoembryonic antigen based on Ag nanoparticle decorated infinite coordination polymer fibres. Biosens Bioelectron 57: 219-225.
- Hayat A, Barthelmebs L, Sassolas A, Marty JL (2012) Development of a novel label-free amperometric immunosensor for the detection of okadaic acid. Anal Chim Acta 724: 92-97.
- Dominguez RB, Hayat A, Sassolas A, Alonso GA, Munoz R, et al. (2012) Automated flow-through amperometric immunosensor for highly sensitive and on-line detection of okadaic acid in mussel sample. Talanta 99: 232-237.
- Kantiani L, Farré M, Barceló D, Barceló D (2009) Analytical methodologies for the detection of β-lactam antibiotics in milk and feed samples. TrAC, Trends Anal Chem 28: 729-744.
- Dempsey E, Diamond D, Collier A (2004) Development of a biosensor for endocrine disrupting compounds based on tyrosinase entrapped within a poly(thionine) film. Biosens Bioelectron 20: 367-377.
- Conneely G, Aherne M, Lu H, Guilbault GG (2007) Electrochemical immunosensors for the detection of 19-nortestosterone and methyltestosterone in bovine urine. Sensors Actuators B: Chem 121: 103-112.
- Rose A, Nistor C, Emneus J, Pfeiffer D, Wollenberger U (2002) GDH biosensor based off-line capillary immunoassay for alkylphenols and their ethoxylates. Biosens Bioelectron 17: 1033-1043.
- Kim DM, Rahman MA, Do MH, Ban C, Shim YB (2010) An amperometric chloramphenicol immunosensor based on cadmium sulfide nanoparticles modified-dendrimer bonded conducting polymer. Biosens Bioelectron 25: 1781-1788.
- Kumar A, Aravamudhan S, Gordic M, Bhansali S, Mohapatra SS (2007) Ultrasensitive detection of cortisol with enzyme fragment complementation technology using functionalized nanowire. Biosens Bioelectron 22: 2138-2144.
- Zhu Y, Son JI, Shim YB (2010) Amplification strategy based on gold nanoparticle-decorated carbon nanotubes for neomycin immunosensors. Biosens Bioelectron 26: 1002-1008.
- Vockenroth IK, Atanasova PP, Knoll W, Koper I, Toby A, et al. (2005) Functional tethered bilayer membranes as a biosensor platform. In: Sensors, IEEE. IEEE, p. 3.
- Oh SY, Cornell B, Smith D, Higgins G, Burrell CJ, et al. (2008) Rapid detection of influenza A virus in clinical samples using an ion channel switch biosensor. Biosens Bioelectron 23: 1161-1165.
- Krishnamurthy V, Monfared SM, Cornell B (2010) Ion-channel biosensors-Part I: construction, operation, and clinical studies. IEEE transactions on nanotechnology 9: 303-312.
- McDermott BM, Rux AH, Eisenberg RJ, Cohen GH, Racaniello VR (2000) Two distinct binding affinities of poliovirus for its cellular receptor. J Biol Chem 275: 23089-23096.
- Achen MG, Roufail S, Domagala T, Catimel B, Nice EC, et al. (2000) Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. Eur J Biochem 267: 2505-2515.
- Catimel B, Weinstock J, Nerrie M, Domagala T, Nice EC (2000) Micropreparative ligand fishing with a cuvette-based optical mirror resonance biosensor. J Chromatogr A 869: 261-273.
- Holaska JM, Black BE, Love DC, Hanover JA, Leszyk J, et al. (2001) Calreticulin is a receptor for nuclear export. J Cell Biol 152: 127-140.
- Nielsen PK, Gho YS, Hoffman MP, Watanabe H, Makino M, et al. (2000) Identification of a major heparin and cell binding site in the LG4 module of the laminin α5 chain. J Biol Chem 275: 14517-14523.
- Chen HM, Clayton AHA, Wang W, Sawyer WH (2001) Kinetics of membrane lysis by custom lytic peptides and peptide orientations in membrane. Eur J Biochem 268: 1659-1669.
- Nakatani K, Sando S, Saito I (2001) Scanning of guanine-guanine mismatches in DNA by synthetic ligands using surface plasmon resonance. Nat Biotechnol 19: 51.
- Scire A, Tanfani F, Saccucci F, Bertoli E, Principato G (2000) Specific interaction of cytosolic and mitochondrial glyoxalase II with acidic phospholipids in form of liposomes results in the inhibition of the cytosolic enzyme only. Proteins: Structure, Function, and Bioinformatics 41: 33-39.
- Long F, Zhu A, Shi H (2013) Recent advances in optical biosensors for environmental monitoring and early warning. Sensors (Basel) 13: 13928-13948.
- Stoop AA, Jespers L, Lasters I, Eldering E, Pannekoek H (2000) High-density mutagenesis by combined DNA shuffling and phage display to assign essential amino acid residues in protein-protein interactions: Application to study structure-function of plasminogen activation inhibitor 1 (PAI-I). J Mol Biol 301: 1135-1147.
- Blaesing F, Weigel C, Welzeck M, Messer W (2000) Analysis of the DNA-binding domain of Escherichia coli DnaA protein. Mol Microbiol 36: 557-569.
- Ellson CD, Gobert-Gosse S, Anderson KE, Davidson K, Erdjument-Bromage H, et al. (2001) PtdIns (3) P regulates the neutrophil oxidase complex by binding to the PX domain of p40phox. Nat Cell Biol 3: 679.
- Cooper MA (2002) Optical biosensors in drug discovery. Nat Rev Drug Discov 1: 515-528.
- Damborský P, Švitel J, Katrlík J (2016) Optical biosensors. Essays Biochem 60: 91-100.
- McDonagh C, Burke CS, MacCraith BD (2008) Optical chemical sensors. Chem Rev 108: 400-422.
- Peng Z, Bang-Ce Y (2006) Small molecule microarrays for drug residue detection in foodstuffs. J Agric Food Chem 54: 6978-6983.
- Lasagni FA, Lasagni AF (2011) Fabrication and characterization in the micro-nano range. Adv Struc Mat 10: 361-377.
- Fernandez F, Hegnerova K, Piliarik M, Sanchez-Baeza F, Homola J, et al. (2010) A label-free and portable multichannel surface plasmon resonance immunosensor for on site analysis of antibiotics in milk samples. Biosens Bioelectron 26: 1231-1238.
- De Picciotto S, Dickson PM, Traxlmayr MW, Marques BS, Socher E, et al. (2016) Design principles for SuCESsFul biosensors: Specific fluorophore/analyte binding and minimization of fluorophore/scaffold interactions. J Mol Biol 428: 4228-4241.
- Lin B, Liu D, Yan J, Qiao Z, Zhong Y, et al. (2016) Enzyme-encapsulated liposome-linked immunosorbent assay enabling sensitive personal glucose meter readout for portable detection of disease biomarkers. ACS Applied Materials & Interfaces 8: 6890-6897.
- Xiang Y, Lu Y (2012) Portable and quantitative detection of protein biomarkers and small molecular toxins using antibodies and ubiquitous personal glucose meters. Anal Chem 84: 4174-4178.
- Yan L, Zhu Z, Zou Y, Huang Y, Liu D, et al. (2013) Target-responsive “sweet” hydrogel with glucometer readout for portable and quantitative detection of non-glucose targets. J Am Chem Soc 135: 3748-3751.
- Xiang Y, Lu Y (2011) Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat Chem 3: 697-703.
- Valentini F, Galache Fernandez L, Tamburri E, Palleschi G (2013) Single walled carbon nanotubes/polypyrrole-GOx composite films to modify gold microelectrodes for glucose biosensors: Study of the extended linearity. Biosens Bioelectron 43: 75-78.
- Liang B, Fang L, Yang G, Hu Y, Guo X, et al. (2013) Direct electron transfer glucose biosensor based on glucose oxidase self-assembled on electrochemically reduced carboxyl graphene. Biosens Bioelectron 43: 131-136.
- Liu Y, Yu D, Zeng C, Miao Z, Dai L (2010) Biocompatible graphene oxide-based glucose biosensors. Langmuir 26: 6158-6160.
- Pundir CS, Chauhan N (2012) Acetylcholinesterase inhibition-based biosensors for pesticide determination: a review. Anal Biochem 429: 19-31.
- Wang B, Takahashi S, Du X, Anzai J (2014) Electrochemical biosensors based on ferroceneboronic acid and its derivatives: A review Biosensors (Basel) 4: 243-256.
- Marrazza G (2014) Piezoelectric biosensors for organophosphate and carbamate pesticides: A review. Biosensors (Basel) 4: 301-317.
- Erden PE, Kilic E (2013) A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 107: 312-323.
- Kim J, Imani S, De Araujo WR, Warchall J, Valdes-Ramirez G, et al. (2015) Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens Bioelectron 74: 1061-1068.
- Harris JM, Reyes C, Lopez GP (2013) Common causes of glucose oxidase instability in in vivo biosensing: A brief review. J Diabetes Sci Technol 7: 1030-1038.
- Ogi H (2013) Wireless-electrodeless quartz-crystal-microbalance biosensors for studying interactions among biomolecules: A review. Proc Jpn Acad Ser B Phys Biol Sci 89: 401-417.
- Peng F, Su Y, Zhong Y, Fan C, Lee ST, et al. (2014) Silicon nanomaterials platform for bioimaging, biosensing, and cancer therapy. Acc Chem Res 47: 612-623.
- Shen MY, Li BR, Li YK (2014) Silicon nanowire field-effect-transistor based biosensors: from sensitive to ultra-sensitive. Biosens Bioelectron 60: 101-111.
- Schneider E, Clark DS (2013) Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens Bioelectron 39: 1-13.
- Kunzelmann S, Solscheid C, Webb MR (2014) Fluorescent biosensors: Design and application to motor proteins. EXS 105: 25-47.
- Oldach L, Zhang J (2014) Genetically encoded fluorescent biosensors for live-cell visualization of protein phosphorylation. Chem Biol 21: 186-197.
- Randriamampita C, Lellouch AC (2014) Imaging early signaling events in T lymphocytes with fluorescent biosensors. Biotechnol J 9: 203-212.
- Li M, Li R, Li CM, Wu N (2011) Electrochemical and optical biosensors based on nanomaterials and nanostructures: A review. Front Biosci (Schol Ed) 3: 1308-1331.
- Zhou Y, Chiu CW, Liang H (2012) Interfacial structures and properties of organic materials for biosensors: An overview. Sensors (Basel) 12: 15036-15062.
- Guo X (2013) Single-molecule electrical biosensors based on single-walled carbon nanotubes. Adv Mater 25: 3397-3408.
- Hutter E, Maysinger D (2013) Gold-nanoparticle-based biosensors for detection of enzyme activity. Trends Pharmacol Sci 34: 497-507.
- Lamprecht C, Hinterdorfer P, Ebner A (2014) Applications of biosensing atomic force microscopy in monitoring drug and nanoparticle delivery. Expert Opin Drug Deliv 11: 1237-1253.
- Sang S, Wang Y, Feng Q, Wei Y, Ji J, et al. (2016) Progress of new label-free techniques for biosensors: a review. Crit Rev Biotechnol 36: 465-481.
- Ghoshdastider U, Wu R, Trzaskowski B, Mlynarczyk K, Miszta P, et al. (2015) Molecular effects of encapsulation of glucose oxidase dimer by graphene. RSC Advances 5: 13570-13578.
- Palanisamy S, Karuppiah C, Chen SM (2014) Direct electrochemistry and electrocatalysis of glucose oxidase immobilized on reduced graphene oxide and silver nanoparticles nanocomposite modified electrode. Colloids Surf B Biointerfaces 114: 164-169.
- Tang J, Tang D, Li Q, Su B, Qiu B, et al. (2011) Sensitive electrochemical immunoassay of carcinoembryonic antigen with signal dual-amplification using glucose oxidase and an artificial catalase. Anal Chim Acta 697: 16-22.
- Teles FRR, Fonseca LP (2008) Trends in DNA biosensors. Talanta 77: 606-623.
- Petanen T, Virta M, Karp M, Romantschuk M (2001) Construction and use of broad host range mercury and arsenite sensor plasmids in the soil bacterium Pseudomonas fluorescens OS8. Microb Ecol 41: 360-368.
- Zhang Y, Zhang K, Ma H (2009) Electrochemical DNA biosensor based on silver nanoparticles/poly(3-(3-pyridyl) acrylic acid)/carbon nanotubes modified electrode. Anal Biochem 387: 13-19.
- Wang J, Polsky R, Xu D (2001) Silver-enhanced colloidal gold electrochemical stripping detection of DNA hybridization. Langmuir 17: 5739-5741.
- Crespilho FN, Ghica ME, Florescu M, Nart FC, Oliveira ON, et al. (2006) A strategy for enzyme immobilization on layer-by-layer dendrimer–gold nanoparticle electrocatalytic membrane incorporating redox mediator. Electrochem Commun 8: 1665-1670.
- Pan C, Hu B, Li W, Sun Y, Ye H, et al. (2009) Novel and efficient method for immobilization and stabilization of β-d-galactosidase by covalent attachment onto magnetic Fe3 O4–chitosan nanoparticles. J Mol Catal B: Enzym 61: 208-215.
- Besanger TR, Chen Y, Deisingh AK, Hodgson R, Jin W, et al. (2003) Screening of inhibitors using enzymes entrapped in sol-gel-derived materials. Anal. Chem. 75: 2382-2391.
- Yang M, Yang Y, Liu Y, Shen G, Yu R (2006) Platinum nanoparticles-doped sol-gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens Bioelectron 21: 1125-1131.
- Kim H, Kwon HS, Ahn J, Lee CH, Ahn IS (2009) Evaluation of a silica-coated magnetic nanoparticle for the immobilization of a His-tagged lipase. Biocatal Biotransform 27:246-253.
- Ahmad M, Pan C, Gan L, Nawaz Z, Zhu J (2009) Highly sensitive amperometric cholesterol biosensor based on Pt-incorporated fullerene-like ZnO nanospheres. J Phys Chem C 114: 243-250.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: The next generation. Cell 144: 646-674.
- Liu X, Zhang J, Yan R, Zhang Q, Liu X (2014) Preparation of graphene nanoplatelet-titanate nanotube composite and its advantages over the two single components as biosensor immobilization materials. Biosens Bioelectron 51: 76-81.
- Jeyapragasam T, Saraswathi R (2014) Electrochemical biosensing of carbofuran based on acetylcholinesterase immobilized onto iron oxide–chitosan nanocomposite. Sensors Actuators B: Chem 191: 681-687.
- Zhao B, Liu Z, Fu W, Yang H (2013) Construction of 3D electrochemically reduced graphene oxide–silver nanocomposite film and application as nonenzymatic hydrogen peroxide sensor. Electrochem Commun 27: 1-4.
- Babu KJ, Zahoor A, Nahm KS, Ramachandran R, Rajan MJ (2014) The influences of shape and structure of MnO2 nanomaterials over the non-enzymatic sensing ability of hydrogen peroxide. J Nanopart Res 16: 2250.
- Xiong P, Gan N, Cui H, Zhou J, Cao Y, et al. (2014) Incubation-free electrochemical immunoassay for diethylstilbestrol in milk using gold nanoparticle-antibody conjugates for signal amplification. Microchimica Acta 181: 453-462.
- Zhang S, Zhang L, Zhang X, Yang P, Cai J (2014) An efficient nanomaterial-based electrochemical biosensor for sensitive recognition of drug-resistant leukemia cells. Analyst 139: 3629-3635.
- Feng R, Zhang Y, Ma H, Wu D, Fan H, et al. (2013) Ultrasensitive non-enzymatic and non-mediator electrochemical biosensor using nitrogen-doped graphene sheets for signal amplification and nanoporous alloy as carrier. Electrochim Acta 97: 105-111.
- Li G, Li T, Deng Y, Cheng Y, Shi F, et al. (2013) Electrodeposited nanogold decorated graphene modified carbon ionic liquid electrode for the electrochemical myoglobin biosensor. J Solid State Electrochem 17: 2333-2340.
- Atay S, Piskin K, Yılmaz F, Cakır C, Yavuz H, et al. (2016) Quartz crystal microbalance based biosensors for detecting highly metastatic breast cancer cells via their transferrin receptors. Analytical Methods 8: 153-161.
- Soares JC, Shimizu FM, Soares AC, Caseli L, Ferreira J, et al. (2015) Supramolecular control in nanostructured film architectures for detecting breast cancer. ACS Applied Materials & Interfaces 7: 11833-11841.
- Ali MA, Solanki PR, Srivastava S, Singh S, Agrawal VV, et al. (2015) Protein functionalized carbon nanotubes-based smart lab-on-a-chip. ACS Applied Materials & Interfaces 7: 5837-5846.
- Johari-Ahar M, Rashidi MR, Barar J, Aghaie M, Mohammadnejad D, et al. (2015) An ultra-sensitive impedimetric immunosensor for detection of the serum oncomarker CA-125 in ovarian cancer patients. Nanoscale 7: 3768-3779.
- Xu TS (2016) Bioconjugation of peroxidase-like nanostructures with natural enzyme for in-situ amplified conductometric immunoassay of tissue polypeptide antigen in biological fluids. Biochem Eng J 105: 36-43.
- Wu MS, Liu Z, Shi HW, Chen HY, Xu JJ (2015) Visual electrochemiluminescence detection of cancer biomarkers on a closed bipolar electrode array chip. Anal Chem 87: 530-537.
- Shi HW, Zhao W, Liu Z, Liu XC, Xu JJ, et al. (2016) Temporal sensing platform based on bipolar electrode for the ultrasensitive detection of cancer cells. Anal Chem 88: 8795-8801.
- Kadimisetty K, Malla S, Sardesai NP, Joshi AA, Faria RC, et al. (2015) Automated multiplexed ECL immunoarrays for cancer biomarker proteins. Anal Chem 87: 4472-4478.
- Thakur MS, Ragavan KV (2013) Biosensors in food processing. J Food Sci Technol 50: 625-641.
- Karentz D, Lutze LH (1990) Evaluation of biologically harmful ultraviolet radiation in Antarctica with a biological dosimeter designed for aquatic environments. Limnol Oceanogr 35: 549-561.
Citation: Rajpoot K (2017) Recent Advances and Applications of Biosensors in Novel Technology. Biosens J 6: 145. Doi: 10.4172/2090-4967.1000145
Copyright: ©2017 Rajpoot K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 22697
- [From(publication date): 0-2017 - Dec 06, 2023]
- Breakdown by view type
- HTML page views: 21273
- PDF downloads: 1424