The Study of the Influence of Unfolded Protein Response and Autophagy on Sorafenib-Induced Tumor Inhibition of Hepatocellular Carcinoma
Manuscript No. CMB-22-56212 / Editor assigned: 07-Mar-2022 / PreQC No. CMB-22-56212 (PQ); / Reviewed: 16-Mar-2022 / QC No. CMB-22-56212 / Revised: 21-Mar-2022 / Manuscript No. CMB-22-56212 (R) / Accepted Date: 21-Mar-2022 / Published Date: 28-Mar-2022 DOI: 10.4172/1165-158X.1000227
Abstract
To study the effects of the unfolded protein response (UPR) activation and autophagy inhibition on sorafenib inducing apoptosis of hepatocellular carcinoma (HCC) cells. We activated UPR of the HepG2 cells by dithiothreitol (DTT) and tunicamycin (Tun). 3-methyladenine was used to inhibit autophagy of HepG2 cells. The proliferation, apoptosis and adhesion ability of HCC cells were tested by MTT assay and flow cytometry, respectively. The invasion and migration abilities of cells were detected by Trans well assay. Moreover, we established HCC orthotopic transplantation tumor model of nude mice. The activation of UPR can inhibit the sorafenib-induced apoptosis of HCC cells. Inhibiting autophagy significantly enhanced the sorafenib-induced apoptosis of HCC cells. Activating the UPR can enhance the sorafenib resistance of HCC cells. On the contrary, inhibiting autophagy causes opposite effects.
Keywords
Hepatocellular carcinoma; Sorafenib; Unfolded protein response; Autophagy
Introduction
Liver cancer is one of the most common malignancies worldwide. In China, the incidence of liver cancer ranks fifth among all malignant tumors and the fatality rate ranks second. Statistics show that about half of the newly diagnosed cases of liver cancer worldwide come from China [1]. According to the relevant prediction of the World Health Organization (WHO), the number of deaths due to liver cancer can reach millions in 2030 [2,3]. Hepatocellular carcinoma (HCC) is the major histological subtype of liver cancer, which is imperceptible at the early stage of the disease, develops rapidly, tends to recur and metastasize, is aggressive and has a poor prognosis, thus the overall survival rate of patients is low [4]. With the continuous development of medical technology, the diagnosis and treatment of liver cancer have been continuously improved [5,6]. For early stage patients, first-line treatment includes hepatectomy, ablation and liver transplantation, and the survival rate after treatment can reach 50%. For patients with advanced disease, other therapeutic strategies such as trans catheter arterial chemoembolization (TACE) are used [7]. However, to date, radical treatment of HCC remains a troublesome problem [6]. With the advent of molecular targeted therapies, researchers have invested more and more attention in the study of the mechanism of tumor progression as an attempt to find better, safe, and effective tumor therapeutic targets [8,9].
In 2006, sorafenib was approved by the Food and Drug Administration (FDA) for the treatment of advanced renal cell carcinoma, and in 2007 for the treatment of advanced HCC, which is currently the only molecular targeted drug for advanced HCC [10, 11]. Sorafenib is a small molecule compounds as well as an oral multiple kinase inhibitors that works mainly by inhibiting the proliferation of tumor cells, inhibiting angiogenesis, and promoting the apoptosis of tumor cells. Sorafenib can inhibit the proliferation of tumor cells by inhibiting the kinase activity of Raf1, B-Raf and Ras/Raf/MEK/ERK signaling pathways; it can inhibit tumor angiogenesis by inhibiting hepatic cytokine receptor (c-kit), Fms-like tyrosine kinase 3 (flt3) and vascular endothelial growth factor receptor (VEGFR2, VEGFR3); sorafenib can also induce apoptosis by reducing the phosphorylation level of eIF4E and the expression level of Mcl-1 in tumor cells [12].
Although it has been widely used in the treatment of HCC and is also of great significance to improve the survival time of patients, most patients suffer from recurrence or even death of HCC due to the development of drug resistance [13-16]. Therefore, in-depth study of the influencing factors of sorafenib efficacy or the mechanism of drug resistance is particularly important to improve its efficacy. Unfolded protein response (UPR) [17] and autophagy [18] are 2 kinds of cell protection mechanism, both of which plays a role in the development of drug resistance [19]. In this study, we examined the effects of UPR activation and autophagy inhibition on the cell apoptosis induced by sorafenib treatment.
Materials and Methods
Cell culture
HepG2 hepatoma cell line was cultured with DMEM medium (containing 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/ mL streptomycin) in an incubator at 37℃ and 5% CO2 with saturated humidity, and the medium was changed every 2 days. Autophagy of cells was inhibited with 3-MA at a concentration of 2 mmol/L, and the UPR was induced with 1.25 mmol/L DTT and 1 μg/mL Tun. The cells were divided into 6 groups: the control group without treatment (Con); the 3-MA group treated with 3-MA; the UTR group treated with
DTT and Tun; the Sora group treated with indicated concentration of sorafenib; the UPR+Sora group treated with both UPR induction and sorafenib treatment; the 3-MA+Sora group treated with 3-MA and sorafenib. Cells in each group were treated for 12 h followed by relevant assays.
MTT assay
Cells in logarithmic growth phase were adjusted to 5 × 104/mL, and were seeded (100 μL in each well) in a 96-well plate and cultured in a cell incubator at 5% CO2 and 37℃ for 12 h. After the cells adhered, the culture medium in each well was discarded, and 100 μL of sorafenib at different experimental concentrations was added, respectively. The control group was added with an equal volume of DMEM culture medium (containing 10% fetal bovine serum), with 6 duplicate wells in each group. Only DMEM culture medium was added to the zero adjustment group without cells. After culture for 24 h, 48 h and 72 h, 20 μL MTT solutions was added to each well, the culture was continued for 4 h, after that, the liquid in the wells was discarded, 150 μL DMSO solution was added to each well, and the plate was shaken on a shaker at low speed for 10 min. The absorbance value was detected at the wavelength of 490 nm of microplate reader. Cell survival rate was calculated by the formula: [(OD value of experimental group - OD value of zeroing group)/(OD value of control group - OD value of zeroing group)] × 100%.
Cell adhesion assay
96-well culture plates were first coated with 20 μL of Matrigel gel (2 μg/50 μL) per well, incubated overnight at 37℃ in a 5% CO2 incubator, and incubated with 2% bovine serum albumin for 1 h until use. They were divided into 6 groups of cells as mentioned before, with 6 duplicate wells in each group; the above cells were seeded in the treated 96-well plate at 5 × 104 cells/well, respectively, incubated for 120 min and then the non-adhered cells in the wells were discarded. After rinsing twice with PBS solution, 20 μL of 5 g/L MTT solution was added, cultured for 4 h and then 150 μL of DMSO was successively added, and the absorbance value was detected at a wavelength of 490 nm on a microplate reader. Calculation of adhesion rate in each group = (mean OD value of treatment group/mean OD value of control group cells) × 100%.
Cell apoptosis assay
HepG2 cells in the logarithmic growth phase, were seeded in 6-well plates at a density of 5 × 105/mL. After the cells adhered, they were grouped as mentioned before. After culture in the cell incubator for 48 h, 1 mL of suspended and adherent cells was collected with a flow tube, centrifuged and washed once with ice-cold PBS. The cells were resuspended with 500 μL of Binding Buffer, added with 5 μL AnnexinVFITC and 10 μL PI, reacted at room temperature for 15 min in the dark, and detected by flow cytometry within 1h.
Transwell assay
The cell density of the 6 groups of cells was adjusted to 2 × 105/ mL with serum-free medium, 100 μL was added to the upper chamber of the transwell, and 500 μL of culture medium containing 10% FBS was added to the lower chamber of the 24-well plate. After 24 h of routine incubation in a cell incubator, cells in the upper chamber were gently rinsed with PBS, and then the cells in the upper chamber were gently wiped off with a cotton swab, fixed with 95% alcohol for 10 min, and stained with 0.1% crystal violet for 15 min. Under an inverted microscope, 5 fields were randomly selected (×100) for observing the number of cells penetrating the insert.
For detecting cell invasion, 70 μL of Matrigel (1:3 dilution) was evenly coated on a transwell upper chamber polycarbon lipid membrane (membrane pore size 8 μm) and placed in a cell incubator at 37 and 5% CO2 overnight to solidify the matrigel. The rest steps were equivalent to the cell migration assay described above.
Orthotopic xenograft model
30 animals were equally and randomly divided into 6 groups with 5 animals in each group. After intraperitoneal injection of 1% pentobarbital sodium for anesthesia, a transverse incision about 1 cm in length was made in the left upper quadrant of the rat to fully expose the left lobe of the liver. 150 μL (concentration of 1 × 107/mL) of HepG2 cells in each experimental group was injected into the liver (generally the left lobe of the liver). The hemostasis was performed by pressing with a sterile cotton swab, and the abdomen was closed layer by layer with 4-0 atraumatic suture. The mice had normal diet after operation, and no antibiotics were applied. After inoculation, the survival status and mental status were observed daily, and 4 weeks later, exploratory laparotomy was performed one by one to macroscopically observe the presence or absence of orthotopic tumor formation in the liver and calculate the tumor volume. Animal study was approved by Medical Ethic Committee of the Second Affiliated Hospital of Harbin Medical University (sydwgzr2021-192).
Tumor volume = (longest tumor diameter) × (shortest tumor diameter)2 / 2
Statistical analysis SPSS 19.0 statistical software was used for analysis. The data were expressed as mean ± standard deviation. The t-test and analysis of variance were used for comparison. P < 0.05 indicated statistically significant difference.
Results
The effects of UPR activation or autophagy suppression on sorafenib-induced inhibition of HCC cell proliferation
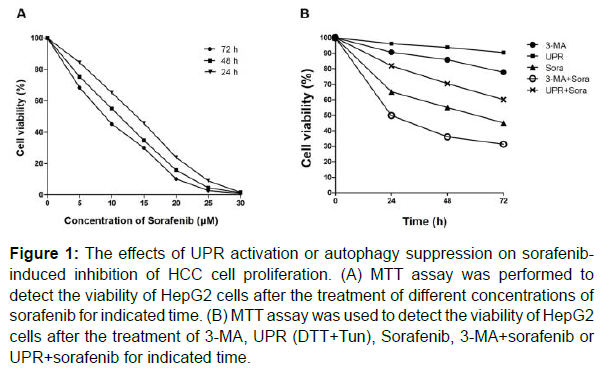
First of all, the effects of sorafenib treatment on HCC cell proliferation were evaluated. The results of MTT assay showed that sorafenib, with different concentrations, inhibited the cell viability of HepG2 cells in a time-dependent manner. The IC50 was 11.19 ± 1.31 μmol/L at 24 h, 9.70 ± 1.48 μ mol/L at 48h, and 8.17 ± 1.37 μmol/L at 72h (Figure 1A). Based on these results, 10 μmol/L sorafenib was selected as the experimental concentration for subsequent study. Then, a combined treatment of sorafenib and UPR activation (DTT+Tun) or autophagy suppression (3-MA) was carried out to assess the altered therapeutic efficiency of sorafenib. As shown in Figure 1B, UPR activation exerted no significant inhibitory effects on HepG2 cell proliferation, but significantly inhibited sorafenib-induced HepG2 cell death, while 3-MA combined with sorafenib group had a more significant inhibitory effect on HepG2 cells than sorafenib group. Collectively, activation of the UPR effectively protected HCC cells from sorafenib, and inhibition of autophagy by 3-MA enhanced the therapeutic efficiency of sorafenib.
Figure 1: The effects of UPR activation or autophagy suppression on sorafenibinduced inhibition of HCC cell proliferation. (A) MTT assay was performed to detect the viability of HepG2 cells after the treatment of different concentrations of sorafenib for indicated time. (B) MTT assay was used to detect the viability of HepG2 cells after the treatment of 3-MA, UPR (DTT+Tun), Sorafenib, 3-MA+sorafenib or UPR+sorafenib for indicated time.
The effects of UPR activation or autophagy suppression on sorafenib-induced change of HCC cell adhesion
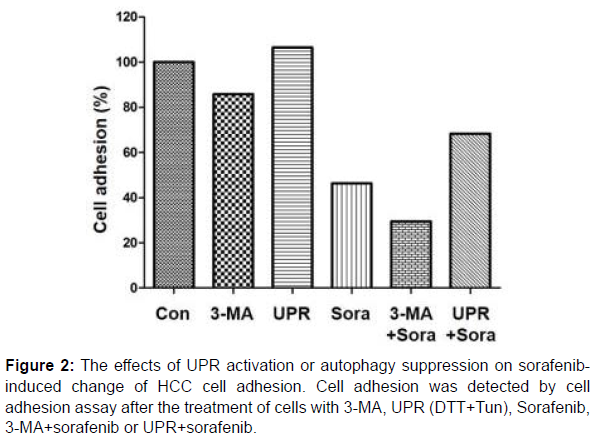
Next, HepG2 cells treated with sorafenib, 3-MA, UPR (DTT+Tun), sorafenib+3-MA or sorafenib+UPR were subjected to the detection of cell adhesion. As shown in Figure 2, sorafenib treatment could significantly reduce the adhesion of HepG2 cells; UPR activation could enhance the adhesion of HepG2 cells and attenuate the effects of sorafenib; the inhibition of autophagy restrained the adhesion of HepG2 cells, which was further strengthened by the combination of sorafenib.
The effects of UPR activation or autophagy suppression on sorafenib-induced change of HCC cell apoptosis
The cell apoptosis upon treatment of different conditions was further examined by flow cytometry. After 48 h of treatment, HepG2 cell apoptosis was 4.3 ± 1.1% in the negative control group, 3.2 ± 0.9% in the UPR group, increased to 8.7 ± 0.7% in the 3-MA group, significantly elevated to 48.9 ± 4.8% in the sorafenib group with dominant early apoptosis. Notably, the sorafenib-induced cell apoptosis could be distinctly alleviated by the activation of UPR (UPR+Sora group) and further aggravated by the inhibition of autophagy (3-MA+Sora group) (Figure 3).
The effects of UPR activation or autophagy suppression on sorafenib-induced change of HCC cell migration and invasion
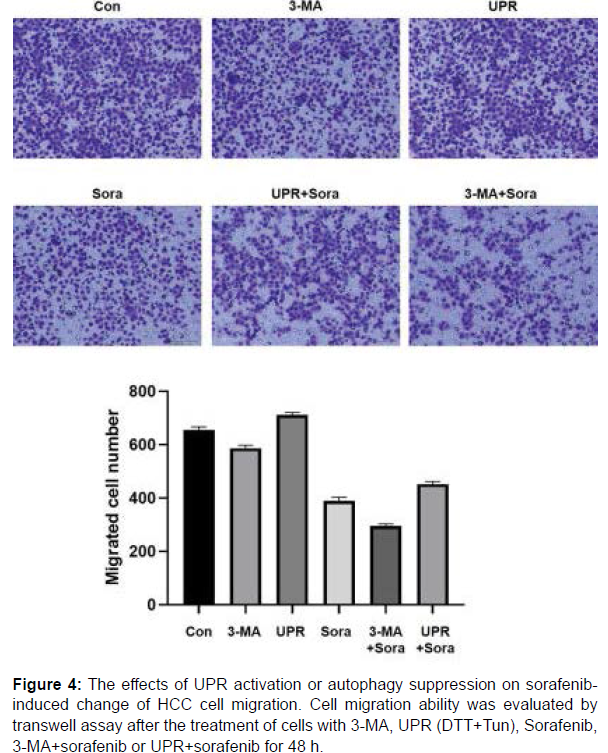
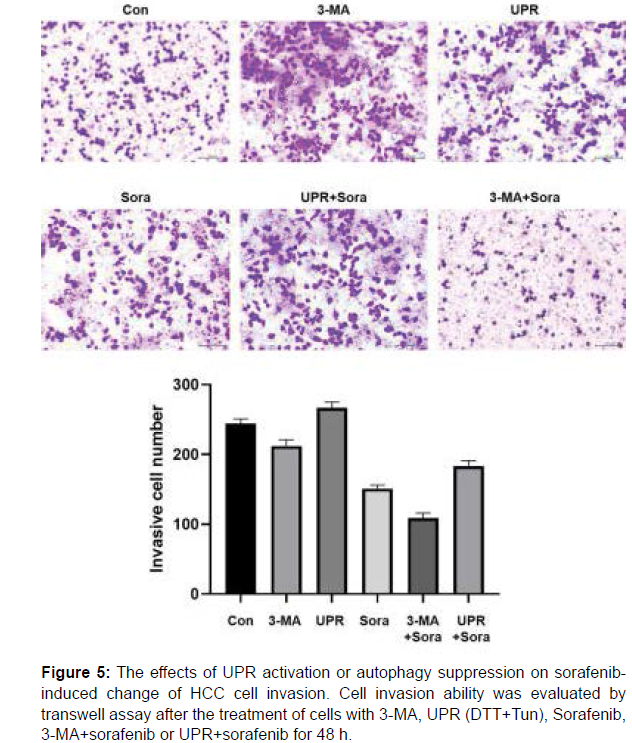
Metastasis is one of the most annoying features of malignant tumors which cause poor prognosis and even the death of patients. Therefore, cell migration and invasion abilities of HepG2 cells in various experimental groups were assessed by transwell assay. As shown in Figure 4 and 5, similar pattern could be observed in both detection of cell migration and invasion, which means the enhancement of cell migration/invasion by UPR, the inhibition of cell migration/invasion by 3-MA and sorafenib to different extents, the alleviation of sorafenibderived change by UPR and the combined enhancement with 3-MA and sorafenib.
The effects of UPR activation or autophagy suppression on sorafenib-induced change of HCC tumor growth in vivo
For verifying the above results in vivo, a total of 30 nude mice were inoculated with 100% tumor survival rate and tumor formation rate. It could be observed that the tumor shape was mostly nodular, the sizes were 0.3 cm × 0.2 cm ~ 1.1 cm × 0.9 cm, and the section of tumor tissue was grayish white or grayish yellow. The tumors from largest to smallest were: UPR group, control group, 3-MA group, UPR+Sora group, Sora group and 3-MA+Sora group. It can be seen that activation of the UPR can promote tumor development, 3-MA or sorafenib treatment can inhibit tumor growth, the combination of sorafenib and 3-MA manifested the strongest inhibitory effect on tumor growth (Figure 6).
Figure 6: The effects of UPR activation or autophagy suppression on sorafenibinduced change of HCC tumor growth in vivo. Cells treated with 3-MA, UPR (DTT+Tun), Sorafenib, 3-MA+sorafenib or UPR+sorafenib were used for constructing orthotopic xenograft mice model for in vivo study. After sacrificing the mice, the xenografts were obtained and the volume was compared among the groups.
Discussion
Currently, surgery is still the first choice and the most effective method for the treatment of HCC. However, HCC tumors of most patients have been developed into the middle or advanced stage when found because the early symptoms and signs are not obvious [20]. Therefore, the surgical effect is unsatisfactory and adjuvant chemotherapy drugs are in urgent need. For a long time, because HCC showed a high resistance too conventional chemotherapeutic drugs, conventional chemotherapeutic drugs have a poor therapeutic efficiency on HCC. In order to further improve the condition of patients with HCC, researchers have been studying the molecular mechanism of HCC, hoping to discover and develop more effective methods and drugs for the treatment of HCC [8].
The endoplasmic reticulum is a model organelle with secretory function, which plays an important role in maintaining the normal physiological function of eukaryotic cells. The endoplasmic reticulum is involved in many intracellular biochemical reactions, mainly including protein synthesis and transport, modification and processing, lipid secretion, detoxification, and maintenance of calcium homeostasis. It should be noted that the ER can only process and transfer correctly folded proteins to the Golgi. When adverse factors such as hypoxia, nutritional deficiency, energy deficiency, oxidative stress, glycosylation changes, calcium consumption, or DNA damage exist, they can cause endoplasmic reticulum stress (ERS), which is a pathophysiological response resulting from the accumulation of unfolded or misfolded proteins in the endoplasmic reticulum [19, 20]. Meanwhile, related cascades, such as the unfolded protein response (UPR) and ERassociated protein degradation (ERAD) were also initiated along with ERS [17]. Among them, UPR is the most important protective signaling pathway in ERS response and plays an important role in metabolism, oxidative stress and inflammatory response [21]. The UPR functions through three ER transmembrane receptor-mediated proteins, which are pancreatic endoplasmic reticulum kinase (PERK), inositol requiring enzyme 1 alpha (IRE-1α), and activating transcription factor 6 (ATF6) [17]. These three transmembrane proteins all contain an ER lumen domain and directly or indirectly sense misfolded proteins, which cause respective transmembrane proteins to oligomerize and phosphorylate themselves and switch to an activated state. The function of the UPR has been extensively studied in diseases such as atherosclerosis, heart disease, amyotrophic lateral sclerosis (ALS) and cancer [22]. In tumors, the activation of UPR can assist tumor cells to avoid the effects of hostile environments to some extent and ensure their survival; it can also promote tumor cells to acquire drug resistance through dormancy and immunosuppression [23-25]. Consistently, our study showed that the activation of UPR could promote cell adhesion, inhibit cell apoptosis, as well as enhance cell migration/invasion and tumor growth in vivo. What’s more, UPR activation could partially reverse the tumor inhibition induced by sorafenib treatment.
The complexity of autophagy and the diversity of their substrates have led to the paradoxical role that autophagy plays in tumor regulation. On the one hand, autophagy plays an anti-tumor role. Autophagy regulates the energy balance, mitochondrial turnover, substance metabolism and other functions of normal tissue cells, maintains genome stability, and prevents mutations from leading to canceration; moreover, autophagy is involved in the balance and function of the immune system, so inhibition of autophagy may lead to impairment of anti-tumor function. On the other hand, autophagy plays an important role in promoting tumors. Because autophagy acts as a self-renewal mechanism in the physiological state of cells and a self-protection mechanism in the face of external stress, its nature of protecting cells is not changed even in tumor cells [26-28]. In tumor cells, autophagy can lead to the resistance of tumor cells to molecular targeted drugs, maintain the genomic stability of tumor cells to prevent immunogenic death, and reduce the recognition ability of the immune system to tumor cells [29, 30]. Inhibition of autophagy can therefore disrupt the energy supply balance of tumor cells, inhibit the metabolic function of mitochondria and produce ROS, and perturb the intracellular nucleotide repertoire, which causes tumor cell damage [31]. Indeed, we found that, the inhibition of autophagy by treatment of 3-MA could reduce the malignant phenotypes of HCC cells and aggravate the sorafenib-induced tumor suppression.
In summary, activation of UPR can enhance the drug resistance of HCC cells and weaken the lethality of sorafenib; inhibition of autophagy can enhance the effect of sorafenib and improve the sensitivity of HCC cells to sorafenib. Therefore, we can get inspiration that the application of UPR blockers and autophagy inhibitors can enhance the therapeutic efficiency of sorafenib in HCC cells.
Acknowledgements
None.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This work was financially supported by Harbin Science and Technology Bureau project (No. 2017RAQXJ198).
Author Contributions
X. Zhang and L. Zhang designed this program. J. Wu and Q. Zhuang operated the cell and animal experiments. X. Gao, X. Yang and R. Qin conducted the data collection and analysis. J. Wu and Q. Zhang produced the manuscript which was checked by X. Zhang. All the authors have confirmed the submission of this manuscript.
Data availability
The data generated in this study are available within the article and its supplementary data files.
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, et al. (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66: 115-132.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424.
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer Statistics, 2021. CA Cancer J Clin 71: 7-33.
- Kulik L, El-Serag HB (2019) Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 156: 477-491.
- Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A (2020) Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 17: 139-152.
- Yang JD, Heimbach JK (2020) New advances in the diagnosis and management of hepatocellular carcinoma. Bmj 371.
- Vogel A, Saborowski A (2020) Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat Rev 82: 101946.
- Ogunwobi OO, Harricharran T, Huaman J, Galuza A, Odumuwagun O, et al. (2019) Mechanisms of hepatocellular carcinoma progression. World J Gastroenterol 25: 2279-2293.
- Alqahtani A, Khan Z, Alloghbi A, Ahmed TSS, Ashraf M, et al. (2019) Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina (Kaunas) 55.
- Cheng Z, Wei-Qi J, Jin D (2020) New insights on sorafenib resistance in liver cancer with correlation of individualized therapy. Biochim Biophys Acta Rev Cancer 1874: 188382.
- Boland P, Wu J (2018) Systemic therapy for hepatocellular carcinoma: beyond sorafenib. Chin Clin Oncol 7: 50.
- Chen F, Fang Y, Zhao R, Le J, Zhang B, et al. (2019) Evolution in medicinal chemistry of sorafenib derivatives for hepatocellular carcinoma. Eur J Med Chem 179: 916-935.
- Adhoute X, Pénaranda G, Raoul JL, Bourlière M (2020) Prognostication of HCC under sorafenib: Is it always possible? Liver Int 40: 1241-1243.
- Xia S, Pan Y, Liang Y, Xu J, Cai X (2020) The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine 51: 102610.
- Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, et al. (2020) The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther 5: 87.
- Cabral LKD, Tiribelli C, Sukowati CHC (2020) Sorafenib Resistance in Hepatocellular Carcinoma: The Relevance of Genetic Heterogeneity. Cancers (Basel) 12.
- Hetz C, Zhang K, Kaufman RJ (2020) Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol 21: 421-438.
- Onorati AV, Dyczynski M, Ojha R, Amaravadi RK (2018) Targeting autophagy in cancer. Cancer 124: 3307-3318.
- Qi Z and Chen L (2019) Endoplasmic Reticulum Stress and Autophagy. Adv Exp Med Biol 1206: 167-177.
- Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, et al. (2019) A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16: 589-604.
- Grootjans J, Kaser A, Kaufman RJ, Blumberg RS (2016) The unfolded protein response in immunity and inflammation. Nat Rev Immunol 16: 469-484.
- Zhang G, Wang X, Gillette TG, Deng Y, Wang ZV (2019) Unfolded Protein Response as a Therapeutic Target in Cardiovascular Disease. Curr Top Med Chem 19: 1902-1917.
- Wang M, Law ME, Castellano RK, Law BK (2018) The unfolded protein response as a target for anticancer therapeutics. Crit Rev Oncol Hematol 127: 66-79.
- Madden E, Logue SE, Healy SJ, Manie S, Samali A (2019) The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance. Biol Cell 111: 1-17.
- Hsu SK, Chiu CC, Dahms HU, Chou CK, Cheng CM, et al. (2019) Unfolded Protein Response (UPR) in Survival, Dormancy, Immunosuppression, Metastasis, and Treatments of Cancer Cells. Int J Mol Sci 20(10): 2518.
- Levy JMM, Towers CG, Thorburn A (2017) Targeting autophagy in cancer. Nat Rev Cancer 17: 528-542.
- Li X, He S, Ma B (2020) Autophagy and autophagy-related proteins in cancer. Mol Cancer 19: 12.
- Kocaturk NM, Akkoc Y, Kig C, Bayraktar O, Gozuacik D, Kutlu O (2019) Autophagy as a molecular target for cancer treatment. Eur J Pharm Sci 134: 116-137.
- Li YJ, Lei YH, Yao N, Wang CR, Hu N, et al. (2017) Autophagy and multidrug resistance in cancer. Chin J Cancer 36: 52.
- Vempati RK, Malla RR (2020) Autophagy-Induced Drug Resistance in Liver Cancer. Crit Rev Oncog 25: 21-30.
- Oakes SA (2020) Endoplasmic Reticulum Stress Signaling in Cancer Cells. Am J Pathol 190: 934-946.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Wu J, Zhuang Q, Gao X, Yang X, Qin R, et al. (2022) The Study of the Influence of Unfolded Protein Response and Autophagy on Sorafenib-Induced Tumor Inhibition of Hepatocellular Carcinoma. Cell Mol Biol, 68: 227. DOI: 10.4172/1165-158X.1000227
Copyright: © 2022 Wu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2082
- [From(publication date): 0-2022 - Dec 06, 2023]
- Breakdown by view type
- HTML page views: 1737
- PDF downloads: 345